
(a)
Interpretation:The denserphase; FCC or BCC on the basis of given P-T curve for potassium needs to be determined.
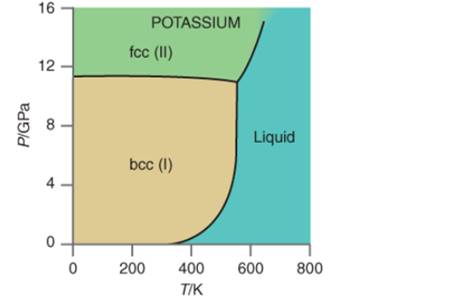
Concept Introduction:All substances can mainly exist in three phases; solid, liquid and gas. These three phases can convert into each other by the application of temperature and pressure such as heating of solid leads to the conversion to liquid and then gaseous
Similarly, the gases can be compressed at high pressure to liquefy. The interconversion of three phases of substance can be shown on the phase diagram.
(b)
Interpretation:The new diagram for different pressure range that indicates the vapor pressure needs to be determined.
Concept Introduction:All substances can mainly exist in three phases; solid, liquid and gas. These three phases can convert into each other by the application of temperature and pressure such as heating of solid leads to the conversion to liquid and then gaseous state of matter.
Similarly, the gases can be compressed at high pressure to liquefy. The interconversion of three phases of a substance can be shownin the phase diagram.
(c)
Interpretation:The possibility of floating or sinking of FCC phase in liquid potassium needs to be explained, if the FCC and liquid potassium are in the equilibrium.
Concept Introduction:All substances can mainly exist in three phases; solid, liquid and gas. These three phases can convert into each other by the application of temperature and pressure such as heating of solid leads to the conversion to liquid and then gaseous state of matter.
Similarly, the gases can be compressed at high pressure to liquefy. The interconversion of three phases of substance can be shown on the phase diagram.
Want to see the full answer?
Check out a sample textbook solution
Chapter 8 Solutions
Physical Chemistry
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





