
Concept explainers
An equimolar liquid mixture of n-pentane and n-hexane at 80°C and 5.00 atm is fed into a flash evaporator at a rate of 100.0 mol/s. When the feed is exposed to the reduced pressure in the evaporator, a substantial amount is vaporized. The temperature in the tank is maintained at 65°C by adding heat. The vapor and liquid phases, which are in equilibrium with each other, are separated and discharged as separate streams. The liquid product stream contains 41.0 mole% pentane. A flowchart and an inlet- outlet enthalpy table for the process are given below.
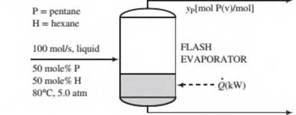
- Using Raoult’s law for vapor-liquid equilibrium calculations, calculate (i) the system pressure, Po(atm), (ii) the mole fraction of pentane in the vapor product, yP, (iii) the volumetric flow rate of the vapor product,
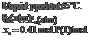 and (iv) the fractional vaporization of pentane, ?(mol vaporized/mol fed).
and (iv) the fractional vaporization of pentane, ?(mol vaporized/mol fed).
 ’s and H’s in the enthalpy table and calculate the required rate of heat addition to the evaporator,
’s and H’s in the enthalpy table and calculate the required rate of heat addition to the evaporator, 
Learn your wayIncludes step-by-step video

Chapter 8 Solutions
ELEMENTARY PRINCIPLES OF CHEM. PROCESS.
Additional Science Textbook Solutions
Process Dynamics and Control, 4e
Elements of Chemical Reaction Engineering (5th Edition) (Prentice Hall International Series in the Physical and Chemical Engineering Sciences)
Problem Solving with C++ (10th Edition)
Data Mining for Business Analytics: Concepts, Techniques, and Applications with XLMiner
Electrical Engineering: Principles & Applications (7th Edition)
Starting out with Visual C# (4th Edition)
- It is desired to determine the molecular weight of an organic compound known to be volatile. For this, a glass balloon with a full volume of 152 mL is weighed and the weighing result is recorded as 215 g. Then, the organic compound that is desired to have some molecular weight in liquid form is added into the balloon and evaporated in a water bath until dryness. After the steam temperature is measured as 90 °C, the balloon is left to cool and the organic compound in the balloon condenses again. The balloon is then weighed again and recorded as 215,367g. Find the molecular weight of this organic compound since the pressure of the environment where the experiment is performed is 700 mmHg.arrow_forwardThe table below shows temperature/composition data collected for a mixture of methylbenzene (M) and octane (O) at 1 atm. Recall that x stands for the mole fraction in the liquid and y stands for the mole fraction in the vapor in equilibrium. The boiling points for methylbenzene (M) and octane (O) are 110.60C and 125.60C, respectively. Construct the phase diagram with Temperature vs. xM. What is the composition of the vapor in equilibrium with the liquid of composition (a) xM = 0.250 and (b) xO = 0.250. T (0C) 110.9 112.0 114.0 115.8 117.3 119.0 121.1 123.0 xM 0.908 0.795 0.615 0.527 0.408 0.300 0.203 0.097 yM 0.923 0.836 0.698 0.624 0.527 0.410 0.297 0.164arrow_forwardA gas mixture contains 10.0 mole% H 2O(v) and 90.0 mole% N 2. The gas temperature and absolute pressure at the start of each of the three parts of this problem are 50°C and 500 mm Hg. Ideal-gas behavior may be assumed in every part of this problem.(a) If some of the gas mixture is put in a cylinder and slowly cooled at constant pressure, at what temperature would the first drop of liquid form?(b) If a 30.0-liter flask is filled with some of the gas mixture and sealed and 70% of the water vapor in the flask is condensed, what volume (cm 3 ) would be occupied by the liquid water? What would be the system temperature?(c) If the gas mixture is stored in a rigid-walled cylinder and a low-pressure weather front moves in and the barometric (atmospheric) pressure drops, which of the following would change: (i) the gas density, (ii) the absolute pressure of the gas, (iii) the partial pressure of water in the gas, (iv)the gauge pressure of the gas, (v) the mole fraction of water in the gas, (vi)…arrow_forward
- L-Serine is an amino acid important for its roles in synthesizing other amino acids and for its use in intravenous feeding solutions. It is often synthesized commercially by fermentation, and recovered by subjecting the fermentation broth to several processing steps and then crystallizing the serine from an aqueous solution. The solubilities of L-serine (L-Ser) in water have been measured at several temperatures, producing the following data:5T(K) 283.4 285.9 289.3 299.1 316.0 317.8 322.9 327.1x(mole fraction L-Ser) 0.0400 0.0426 0.0523 0.0702 0.1091 0.1144 0.1181 0.1248One of the ways such data can be represented is with the van’t Hoff equation: ln x =( a=T b).Graph the data so that the resulting plot is linear. Estimate a and b and give their units.arrow_forwardA single effect evaporator is used to concentrate 10 000 kg / hr of tomato juice from 5% total solids to 30% total solids. The juice enters the evaporator at 15 ° C. The evaporator is operated on Steam (80% quality) at 143.27 kPa. The vacuum in the evaporator allows the juice to boil at 75 ° C. The specific heat of the dilute material is 4.1 kJ / (kg ° C) and the concentrate product is 3.1 kJ / (kg ° C). Count it (a) Steam demand rate = Answer kg / hour. b. Steam economy when condensate temperature is released at 75 ° C. = Answer (kg of water evaporated / kg of steam)arrow_forwardTwo closed tanks are connected to each other by a valve. The first tank contains oxygen (O₂, m= 2.4 kg, T= 134 °C, p= 5 bar) and the other carbon dioxide (CO₂, m= 2.4 kg, T = 33 °C, p = 1.0 bar). When the valve is opened, the gases are allowed to mix. When the mixture reaches equilibrium, the temperature of the mixture is 73 °C. The gases can be assumed to be ideal gases. Calculate m³ (two decimal accuracy) 1) Total volume of the tanks 2) Final pressure of the mixture 3) Molar fraction of oxygen in the mixture 4) Molar fraction of carbon dioxide in the mixture 5) Partial pressure of oxygen in the mixture 6) Partial pressure of carbon dioxide in the mixture 7) Average specific heat capacity of oxygen at constant volume 8) Average specific heat capacity of carbon dioxide at constant volume 9) Heat transferred from or to the process kPa (zero decimal accuracy) % (zero decimal accuracy) % (zero decimal accuracy) kPa (zero decimal accuracy) kPa (zero decimal accuracy) kJ/kgk (three decimal…arrow_forward
- A mixture of 87.3 mole % methanol and 12.7 mole % isopropyl alcohol is vaporized at 101.325 kPa (absolute) until 900 moles of vapor and 100 moles of liquid in equilibrium with each other are produced. This occurs in a single-stage system and the vapor and liquid are kept in contact until equilibrium is achieved. Calculate the composition of the vapor and liquid. Note: Assume the system exhibits ideal behavior (which it mostly does), e.g. Raoult’s Law applies and an Antoine’s Vapor Pressure correlation is a sufficiently accurate model of component vapor pressure.arrow_forwardConsider the following reaction CS2(9) + 302(9) – CO2(9) + 2SO2(g) A mixture containing only CS2(g) and excess O2(g) at a total pressure of 100 kPa is placed in a sealed vessel. After the reaction is completed and the vessel is cooled to the initial temperature, the total pressure in the vessel drops to 80 kPa. What was the mole fraction of CS2(g) in the initial mixture? A. 0.80 B. 0.50 C. 0.20 D. 0.75 E. 0.25arrow_forwardWhat is the flow rate?arrow_forward
- 2-E, A liquid mixture of 30 mol benzene. 30 mol toluene, and 40 mol water initially at 70°C and 101.3 kPa total pressure is heated slowly at a constant pressure of 101.3 kPa to 90°C. The vapor generated stays in contact with the remaining liquid. Assuming equilibrium between phases at all times. estimate (a) the temperature at which vaporization begins, (b) the composition of the first vapor. (c) the temperature at which vaporization is complete, and (d) the composition of the last liquid. Note: Water is cssentially totally immiscible with benzene and toluene. Each liquid phase contributes to the total vapor pressure. Over the temperature range involved, the vapor pressure of benzene is 2.60 times that of toluene, and the vapor pressure of water is 1.23 times that of toluene. Vapor pressure of water is: T. C 70 | 72 | 74 78 | 80 | 82 | 84 | 86 76 88 90 p, kPa 31.2 34.0 36.9 40.1 43.6 47.3 51.3 55.6 60.1 64.9 70.1arrow_forwardWhat is the change in the boiling point of water at 1000 C per Pa change under atmospheric pressure conditions? The molar enthalpy of vaporization is 40.69 kJ mol-1, the molar volume of liquid water is 0.019 x 10-3 m3 mol-1, and the molar volume of steam is 30.199 x 10-3m3 mol-1, all at 100.000 C and 1.01325 bar. (Hint: change in temperature per change in pressure, dT/dP).arrow_forward7.55. A liquid mixture of benzene and toluene is to be separated in a continuous single-stage equilibrium flash tank. Vapor product ny(mol) YB(mol C,H/mol) Liquid feed I mol (basis) T(°C) P(mm Hg) ZB (mol C,H/mol) (1 – zg)(mol C,Hg/mol) 130°C Liquid product n (mol) Xg(mol CgH/mol) Heat The pressure in the unit may be adjusted to any desired value, and the heat input may similarly be adjusted to vary the temperature at which the separation is conducted. The vapor and liquid product streams both emerge at the temperature T(°C) and pressure P(mm Hg) maintained in the vessel. Assume that the vapor pressures of benzene and toluene are given by the Antoine equation, Table B.4 or APEX; that Raoult's law–Equation 6.4-1¬applies; and that the enthalpies of benzene and toluene liquid and vapor are linear functions of temperature. Specific enthalpies at two tempera- tures are given here for each substance in each phase. C,H6(1) (T = 0°C, Ĥ = 0 kJ/mol) C,H6(v) (T = 80°C, Ĥ = 41.61 kJ/mol) (T =…arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





