
Polyvinylpyrrolidone (PVP) is a polymer product used as a binding agent in pharmaceutical applications as well as in personal-care items such as hairspray. In the manufacture of PVP, a spray-drying process is used to collect solid PVP from an aqueous suspension, as shown in the flowchart on the next page. A liquid solution containing 65 wt% PVP and the balance water at 25°C is pumped through an atomizing nozzle at a rate of 1500 kg/h into a stream of preheated air flowing at a rate of 1.57 × 104 SCMH. The water evaporates into the stream of hot air and the solid PVP particles are suspended in the humidified air. Downstream, the particles are separated from the air with a filter and collected. The process is designed so that the exiting solid product and humid air are in thermal
equilibrium with each other at 110°C. For convenience, the spray-drying and solid-separation processes are shown as one unit that may be considered adiabatic.
| Polymer solution25°C | |||
| Humid Air | |||
| 1IO°C | |||
| SPRAY | |||
Air, 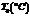 |
DRYER and | Dry solid polymer | |
| SOLID | |||
| SEPARATOR | 110°C | ||
- Draw and completely label the process flow diagram and perform a degree-of-freedom analysis.
Trending nowThis is a popular solution!

Chapter 8 Solutions
ELEMENTARY PRINCIPLES OF CHEM. PROCESS.
Additional Science Textbook Solutions
Process Dynamics and Control, 4e
Elements of Chemical Reaction Engineering (5th Edition) (Prentice Hall International Series in the Physical and Chemical Engineering Sciences)
Fundamentals of Heat and Mass Transfer
ANALYSIS+DESIGN OF LINEAR CIRCUITS(LL)
Introduction to Programming Using Visual Basic (10th Edition)
Starting Out with C++ from Control Structures to Objects (8th Edition)
- You have analyzed an aqueous ammonia solution and find that it contains 30 wt% NH 3.(a) to determine the mass fraction of NH 3 in the vapor that would be in equilibrium with this solution in a closed flask at 1 atm and the corresponding system temperature.(b) If the liquid phase in Part (a) accounts for 90% of the total system mass, calculate the overall system composition and specific enthalpy using balances.arrow_forwardTwo tanks are connected by a series of pipes as shown below. Tank 1 initially contains 150 grams of salt dissolved in 20 liters of water, while tank 2 contains 50 grams of salt dissolved in 10 liters of water. Beginning at time t = 0, pure water is pumped into tank 1 at a volumetric flow rate of 3 liters per minute. This causes brine to flow between the tanks, and to flow out of both tanks at the volumetric flow rates shown in the Fig. 2. Determine the salt concentration in each tank (C, and C2) at any time t>0.arrow_forward6. Assume you have 1kg of an alloy consisting of 85 wt% Sn and 15 wt% Pb at a temperature of 184 °C. How much Sn (in kg) needs to be added to solidify the entire mixture without lowering the temperature? Composition (at% Sn) 20 40 60 80 100 327°C Temperature (°C) 300 200 100 (Pb) a + L 18.3 20 183°C a + B Liquid 61.9 40 60 Composition (wt% Sn) B+L 80 232°C 97.8 B al 600 500 400 300 200 100 100 (Sn) Temperature (°F)arrow_forward
- A mixture of phenol and water, under certain conditions of temperature and composition, forms two separate liquid phases, one rich in phenol and the other rich in water. At 30⁰ the compositions of the upper and lower layers are 70% and 9% by mass phenol, respectively. If 40kg of phenol and 60kg of water are mixed and the layers are allowed to separate at 30⁰C, what will be the weight of the two layers?arrow_forwardThe need arises in a laboratory for 5000cm3 of an antifreeze solution consisting of 50mol% of HCl in water. What volumes of pure HCI and pure water at 25°c must be mixed to form 5000cm3 of antifreeze, also at 25°c partial molar volume of HCl and water in 50 mol% HCl solution and pure species volume, both at 25°c are: HCI : V 1=25.8956 cm³/mol , v1=75.8963 cm³/mol Water: 2 =42.5632 cm³/mol , v2=25.2362 cm3/mol.----- V --- (b). Prove Cp-Cy = R--------arrow_forwardA single effect evaporator is used to concentrate 10 000 kg / hr of tomato juice from 5% total solids to 30% total solids. The juice enters the evaporator at 15 ° C. The evaporator is operated on Steam (80% quality) at 143.27 kPa. The vacuum in the evaporator allows the juice to boil at 75 ° C. The specific heat of the dilute material is 4.1 kJ / (kg ° C) and the concentrate product is 3.1 kJ / (kg ° C). Count it (a) Steam demand rate = Answer kg / hour. b. Steam economy when condensate temperature is released at 75 ° C. = Answer (kg of water evaporated / kg of steam)arrow_forward
- A hydrocarbon stream composed of 40 mol% n-butane and 60 mol% n-octane is fed at 100 kmol/h to a flash vessel operating at 5 bar and 145°C. The liquid outlet stream of the flash vessel is then sent to a distillation column with a partial condenser to further separate the components. 90% of the butane in the feed to the column is recovered in the overhead vapor product (distillate), along with small amounts of octane. The bottoms product contains octane which accounts for 75% of the octane in the fresh hydrocarbon feed (to the flash vessel). The condenser and reboiler operating temperatures are 136.7°C and 165.7°C, respectively. Determine the compositions of the liquid outlet stream of the condenser and the vapor outlet stream of the reboiler.arrow_forwardQ4/in a two stage process, acetic acid (A)is extracted from water (W) into hexanol (H) in a liquid-liquid extraction vessel and the extract is subsequently separated by distillation. Assume that water is completely insoluble in hexanol. A mixture of 18wt% acetic acid and the balance water is feed to liquid-liquid extraction vessel. Pure hexanol is feed to the column to extracted the acetic acid. The water-rich stream leaving the vessel is 99.5wt% water and the balance acetic acid. The hexanol-rich extract from the vessel is feed to a distillation column. The composition of the distillate is 96wt% acetic acid and the balance hexanol. The bottom stream contains 97.2wt% hexanol and recovers 95% of the hexanol feed to the liquid-liquid extraction vessel. Calculate the percentage of acetic acid in the process feed that is recovered in the distillate stream. Distillation Liq-liq Column Extractionarrow_forwardTetracycline produced in Streptomyces aureus fermentations is purified by crystallisation. One hundred kg of a supersaturated solution containing 7.7 wt% tetracycline is cooled in a batch fluidised-bed crystalliser. Seed crystals of tetracycline are added at a concentration of 40 ppm to promote crystal growth. At the end of the crystallisation process, the remaining solution contains 2.8% tetracycline. (a) What is the mass of the residual tetracycline solution? (b) What mass of tetracycline crystals is produced?arrow_forward
- A 3.1 kg mass of saturated liquid-vapor mixture of water is contained within a closed system in a vertically arranged piston-cylinder device at a pressure of P1. Initially, 1.1067 kg of the water is in the liquid phase and the rest is in the vapor phase. Heat is now transferred to the water, and the piston, which is resting on a set of stops, starts moving when the pressure inside reaches P2. Heat transfer continues until the total volume increases by 19 percent. Consider Case #7 from Table 1: Table 1: Case # along with the list of pressures P, and P2 Case # P1 (kPa) P2 (kPa) 100 300 150 300 3 200 500 4 250 500 300 500 350 700 400 700 8. 450 700 (a) show the process on a P-v diagram in reference to the saturation lines, (b) determine the initial and final temperatures, (c) determine the mass of liquid water when the piston first starts moving, and (d) determine the work done during this process.arrow_forwardA gas mixture contains 10.0 mole% H 2O(v) and 90.0 mole% N 2. The gas temperature and absolute pressure at the start of each of the three parts of this problem are 50°C and 500 mm Hg. Ideal-gas behavior may be assumed in every part of this problem.(a) If some of the gas mixture is put in a cylinder and slowly cooled at constant pressure, at what temperature would the first drop of liquid form?(b) If a 30.0-liter flask is filled with some of the gas mixture and sealed and 70% of the water vapor in the flask is condensed, what volume (cm 3 ) would be occupied by the liquid water? What would be the system temperature?(c) If the gas mixture is stored in a rigid-walled cylinder and a low-pressure weather front moves in and the barometric (atmospheric) pressure drops, which of the following would change: (i) the gas density, (ii) the absolute pressure of the gas, (iii) the partial pressure of water in the gas, (iv)the gauge pressure of the gas, (v) the mole fraction of water in the gas, (vi)…arrow_forwardI need a detailed solution for thisarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





