
Organic Chemistry
7th Edition
ISBN: 9780321803221
Author: Paula Y. Bruice
Publisher: Prentice Hall
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 7.4, Problem 10P
What orbitals are used to form the carbon-carbon σ bond between the highlighted carbons?
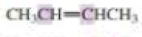


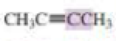

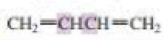
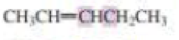
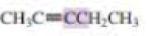

Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
which c-h bond has the lowest BDE (bond disassociation energy)?
Draw an expanded structural formula of pent-1-en-3-yne/ CH3-CC-CH=CH2 and then label each carbon. Indicate the longest and shortest C-H bond and predict the C—C single bond that has the highest BDE(bond dissociation energy).
Draw the skeletal structure of each product for each reaction.
Chapter 7 Solutions
Organic Chemistry
Ch. 7.1 - What is the molecular formula for a monocyclic...Ch. 7.1 - Prob. 2PCh. 7.1 - Draw the structure and give the common and...Ch. 7.1 - Prob. 4PCh. 7.1 - Name the following:Ch. 7.2 - Prob. 6PCh. 7.2 - Name the following:Ch. 7.3 - Prob. 8PCh. 7.3 - Why does cis-2-butene have a higher boiling point...Ch. 7.4 - What orbitals are used to form the carbon-carbon ...
Ch. 7.6 - Prob. 12PCh. 7.6 - Prob. 13PCh. 7.7 - Prob. 14PCh. 7.7 - Which alkyne should be used for the synthesis of...Ch. 7.7 - Prob. 16PCh. 7.8 - Prob. 17PCh. 7.8 - Only one alkyne forms an aldehyde when it...Ch. 7.9 - Describe the alkyne you should start with and the...Ch. 7.9 - Prob. 20PCh. 7.10 - Prob. 21PCh. 7.10 - Prob. 22PCh. 7.10 - Rank the following from strongest base to weakest...Ch. 7.12 - Prob. 26PCh. 7 - What is the major product obtained from the...Ch. 7 - Draw a condensed structure for each of the...Ch. 7 - A student was given the structural formula of...Ch. 7 - Prob. 30PCh. 7 - What is each compounds systematic name?Ch. 7 - What reagents should be used to carry out the...Ch. 7 - Prob. 33PCh. 7 - Prob. 34PCh. 7 - Prob. 35PCh. 7 - Prob. 36PCh. 7 - What is the major product of the reaction of 1 mol...Ch. 7 - What is each compounds systematic name? a....Ch. 7 - What is the molecular formula of a hydrocarbon...Ch. 7 - Answer Problem 39, parts a-b, using 2-butyne as...Ch. 7 - Prob. 41PCh. 7 - a. Starting with 3-methyl 1-butyne, how can you...Ch. 7 - Prob. 43PCh. 7 - Which of the following pairs are keto-enol...Ch. 7 - Prob. 45PCh. 7 - Do the equilibria of the following acid-base...Ch. 7 - What stereoisomers are obtained when 2-butyne...Ch. 7 - Draw the keto tautomer for each of the following:Ch. 7 - Show how each of the following compounds can be...Ch. 7 - A chemist is planning to synthesize 3-octyne by...Ch. 7 - Prob. 51PCh. 7 - What stereoisomers are obtained from the following...Ch. 7 - Prob. 53PCh. 7 - Prob. 54PCh. 7 - Prob. 55PCh. 7 - Prob. 56PCh. 7 - Prob. 57P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which of the following structures are identical? (Green = Cl.)arrow_forwardNH₂ Based 1 2. Select the IUPAC name corresponding to the aromatic compound on the molecular structure and orbital diagram inarrow_forwardMatch the structures to its IUPAC name. 1 5 ОН ОН Br Br С 2 NH2 6 ОН 3 та you 7 8 ОН ОН Brarrow_forward
- Label all carbons as C, CH, CH2, CH3 in the line structure of diosmin i.e, a flavonoid natural product and dietary supplement utilized to treat venous diseases or hemorrhoids. OH HO HO ÓH HO HO. ОН О ОН Diosminarrow_forward3. Some students also confuse the structure of cyclohexanol with that of a phenol. Both structures have a six-carbon ring with a hydroxyl group attached. Use your learnings from the CHM 124 lecture course to explain how the six-carbon rings of cyclohexanol and phenol are different from one another.arrow_forwardDraw propyne and fill in all H’s .arrow_forward
- Identify the number of lone pairs on each highlighted nitrogen atom in the following structure. H. O NH2 H NH2arrow_forwardChemistry In enol form Keto form O C6H5 C6H5 H keto form keto form please show electron arrow. OH enol form OH O enol formarrow_forwardWith reference to structure A, label structure B as an identical compound, a constitutional isomer, a resonance structure, or none of the above. Select the single best answer.arrow_forward
- For the cation shown, four resonance structures are possible. Two resonance forms are given, but they are incomplete. Complete structures 1 and 2 by adding nonbonding electrons and formal charges. Complete the two remaining resonance structures according to their description, including nonbonding electrons and formal charges. Structure 1: add lone pairs and charges X H₂C H₂C CH3 CH₂ Structure 2: add lone pairs and charges H₂C H₂C 0- CH₂ CH₂arrow_forwardPeriodic Table Calculator 7 of 24 Chemistry: Fundamentals and Principles preserted by Saplng Learning Vanillin, CeHeO3, is the active ingredient in vanilla flavoring. It contains a six-membered aromatic ring with an aldehyde group on carbon 1, an alkoxy group (ether) on carbon 3, and a hydroxyl group (alcohol) on carbon 4. Draw the structure of vanillin. e Previous Check Answer Next Hint about us careers privacy policy terms 2019 End of Seaso.doc lyparrow_forwardstructure name CH, — сH,— CH,— С -ОН ОН н н н— С- С — С— Н ннн н н H Н— С — С - CH; H - - Oarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
07 Physical Properties of Organic Compounds; Author: Mindset;https://www.youtube.com/watch?v=UjlSgwq4w6U;License: Standard YouTube License, CC-BY