
Concept explainers
(a)
Interpretation: For the given set of molecules the molecular geometry around the central metal should be predicted using VSEPR model.
Concept Introduction:
Molecular geometry: It is defined as unique three dimensional arrangements of atoms around the central metal present in the molecule which is determined by using spectroscopic techniques and also by using Lewis structure or the valence shell electron pair repulsion theory (VSEPR).
VSEPR Theory:
As the name itself indicates that the basis for this theory is the electron pair that is bonded electron present in either single or double bonds or lone pair electrons, present in the valence shell tends to repel each other which then the tends to be in position in order to minimize the repulsions. The steps involved in the theory in describing the geometry is as follows,
- The first step is to draw the correct Lewis structure for the molecule.
- Then, the electron domain around the central atom should be counted and the geometry that matches with that type of domain in VSEPR should be determined.
- Finally, the geometry is predicted by using the orientation of atoms.
The molecules with considering the domains of type
Lewis structure for any molecule is drawn by using the following steps,
First the skeletal structure for the given molecule is drawn then the total number of valence electrons for all atoms present in the molecule is determined
The next step is to subtract the electrons present in the total number of bonds present in the skeletal structure of the molecule with the total valence electrons such that considering each bond contains two electrons with it.
Finally, the electrons which got after subtractions has to be equally distributed such that each atom contains eight electrons in its valence shell.
Electron Domain: In VSEPR theory, both the lone pair and the bonded pair are together considered as electron domain regardless of the type of bond in which the bonded pair presents.
(a)
Answer to Problem 7.6QP
(a)
Tetrahedral geometry
Explanation of Solution
To predict: The geometry for the given molecule.
Draw the Lewis structure for the molecule (a)
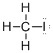
First the skeletal structure for the given molecule is drawn then the total number of valence electrons in the molecule is 14.
The next step is to subtract the electrons present in the total number of bonds present in the molecule with the total valence electrons such that 8 has to be subtracted with 14 as each bond contains two electrons with it and there are four bonds in the skeletal structure.
Finally, the 6 electrons got after subtractions has to be equally distributed such that each atom contains eight electrons in its valence shell.
Determine the molecular geometry for the molecule (a) using VSEPR.
The electron domain for the given molecule is obtained by viewing the Lewis structure which is of type tetrahedral that is four atoms gets bonded with the central atom in the given molecule.
There exist no lone pair on carbon central atom then the molecular geometry for this molecule is tetrahedral.
(b)
Interpretation: For the given set of molecules the molecular geometry around the central metal should be predicted using VSEPR model.
Concept Introduction:
Molecular geometry: It is defined as unique three dimensional arrangements of atoms around the central metal present in the molecule which is determined by using spectroscopic techniques and also by using Lewis structure or the valence shell electron pair repulsion theory (VSEPR).
VSEPR Theory:
As the name itself indicates that the basis for this theory is the electron pair that is bonded electron present in either single or double bonds or lone pair electrons, present in the valence shell tends to repel each other which then the tends to be in position in order to minimize the repulsions. The steps involved in the theory in describing the geometry is as follows,
- The first step is to draw the correct Lewis structure for the molecule.
- Then, the electron domain around the central atom should be counted and the geometry that matches with that type of domain in VSEPR should be determined.
- Finally, the geometry is predicted by using the orientation of atoms.
The molecules with considering the domains of type
Lewis structure for any molecule is drawn by using the following steps,
First the skeletal structure for the given molecule is drawn then the total number of valence electrons for all atoms present in the molecule is determined
The next step is to subtract the electrons present in the total number of bonds present in the skeletal structure of the molecule with the total valence electrons such that considering each bond contains two electrons with it.
Finally, the electrons which got after subtractions has to be equally distributed such that each atom contains eight electrons in its valence shell.
Electron Domain: In VSEPR theory, both the lone pair and the bonded pair are together considered as electron domain regardless of the type of bond in which the bonded pair presents.
(b)
Answer to Problem 7.6QP
(b)
Trigonal planar
Explanation of Solution
To predict: The geometry for the given molecule.
Draw the Lewis structure for the molecule (b)
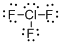
First the skeletal structure for the given molecule is drawn then the total number of valence electrons in the molecule is 28.
The next step is to subtract the electrons present in the total number of bonds present in the molecule with the total valence electrons such that 6 has to be subtracted with 28 as each bond contains two electrons with it and there are three bonds in the skeletal structure.
Finally, the 22 electrons got after subtractions has to be equally distributed such that each atom contains eight electrons in its valence shell.
Determine the molecular geometry for the molecule (b) using VSEPR.
The electron domain for the given molecule is obtained by viewing the Lewis structure which is of type trigonal bipyramidal that is the chlorine atom contains three fluorine atoms and two lone pair of electrons with it hence the geometry for the molecule is T-shaped.
(c)
Interpretation: For the given set of molecules the molecular geometry around the central metal should be predicted using VSEPR model.
Concept Introduction:
Molecular geometry: It is defined as unique three dimensional arrangements of atoms around the central metal present in the molecule which is determined by using spectroscopic techniques and also by using Lewis structure or the valence shell electron pair repulsion theory (VSEPR).
VSEPR Theory:
As the name itself indicates that the basis for this theory is the electron pair that is bonded electron present in either single or double bonds or lone pair electrons, present in the valence shell tends to repel each other which then the tends to be in position in order to minimize the repulsions. The steps involved in the theory in describing the geometry is as follows,
- The first step is to draw the correct Lewis structure for the molecule.
- Then, the electron domain around the central atom should be counted and the geometry that matches with that type of domain in VSEPR should be determined.
- Finally, the geometry is predicted by using the orientation of atoms.
The molecules with considering the domains of type
Lewis structure for any molecule is drawn by using the following steps,
First the skeletal structure for the given molecule is drawn then the total number of valence electrons for all atoms present in the molecule is determined
The next step is to subtract the electrons present in the total number of bonds present in the skeletal structure of the molecule with the total valence electrons such that considering each bond contains two electrons with it.
Finally, the electrons which got after subtractions has to be equally distributed such that each atom contains eight electrons in its valence shell.
Electron Domain: In VSEPR theory, both the lone pair and the bonded pair are together considered as electron domain regardless of the type of bond in which the bonded pair presents.
(c)
Answer to Problem 7.6QP
(c)
Bent shaped
Explanation of Solution
To predict: The geometry for the given molecule.
Draw the Lewis structure for the molecule (c)
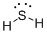
First the skeletal structure for the given molecule is drawn then the total number of valence electrons in the molecule is 8.
The next step is to subtract the electrons present in the total number of bonds present in the molecule with the total valence electrons such that 4 has to be subtracted with 8 as each bond contains two electrons with it and there are two bonds in the skeletal structure.
Finally, the 4 electrons got after subtractions has to be equally distributed over Sulphur atom such that each atom contains eight electrons in its valence shell.
Determine the molecular geometry for the molecule (c) using VSEPR.
The electron domain for the given molecule is obtained by viewing the Lewis structure which is of type tetrahedral since two lone pair of electrons present over Sulphur atom and the molecular geometry for the molecule is bent.
(d)
Interpretation: For the given set of molecules the molecular geometry around the central metal should be predicted using VSEPR model.
Concept Introduction:
Molecular geometry: It is defined as unique three dimensional arrangements of atoms around the central metal present in the molecule which is determined by using spectroscopic techniques and also by using Lewis structure or the valence shell electron pair repulsion theory (VSEPR).
VSEPR Theory:
As the name itself indicates that the basis for this theory is the electron pair that is bonded electron present in either single or double bonds or lone pair electrons, present in the valence shell tends to repel each other which then the tends to be in position in order to minimize the repulsions. The steps involved in the theory in describing the geometry is as follows,
- The first step is to draw the correct Lewis structure for the molecule.
- Then, the electron domain around the central atom should be counted and the geometry that matches with that type of domain in VSEPR should be determined.
- Finally, the geometry is predicted by using the orientation of atoms.
The molecules with considering the domains of type
Lewis structure for any molecule is drawn by using the following steps,
First the skeletal structure for the given molecule is drawn then the total number of valence electrons for all atoms present in the molecule is determined
The next step is to subtract the electrons present in the total number of bonds present in the skeletal structure of the molecule with the total valence electrons such that considering each bond contains two electrons with it.
Finally, the electrons which got after subtractions has to be equally distributed such that each atom contains eight electrons in its valence shell.
Electron Domain: In VSEPR theory, both the lone pair and the bonded pair are together considered as electron domain regardless of the type of bond in which the bonded pair presents.
(d)
Answer to Problem 7.6QP
(d)
Trigonal planar
Explanation of Solution
To predict: The geometry for the given molecule.
Draw the Lewis structure for the molecule (d)
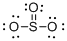
First the skeletal structure for the given molecule is drawn then the total number of valence electrons in the molecule is 24.
The next step is to subtract the electrons present in the total number of bonds present in the molecule with the total valence electrons such that 8 has to be subtracted with 24 as each bond contains two electrons with it and there are four bonds in the skeletal structure.
Finally, the 16 electrons got after subtractions has to be equally distributed over oxygen atoms such that each atom contains eight electrons in its valence shell.
Determine the molecular geometry for the molecule (d) using VSEPR.
The electron domain for the given molecule is obtained by viewing the Lewis structure which is of type trigonal planar and the molecular geometry is also trigonal planar since there are no lone pair of electrons over the central Sulphur atom.
(e)
Interpretation: For the given set of molecules the molecular geometry around the central metal should be predicted using VSEPR model.
Concept Introduction:
Molecular geometry: It is defined as unique three dimensional arrangements of atoms around the central metal present in the molecule which is determined by using spectroscopic techniques and also by using Lewis structure or the valence shell electron pair repulsion theory (VSEPR).
VSEPR Theory:
As the name itself indicates that the basis for this theory is the electron pair that is bonded electron present in either single or double bonds or lone pair electrons, present in the valence shell tends to repel each other which then the tends to be in position in order to minimize the repulsions. The steps involved in the theory in describing the geometry is as follows,
- The first step is to draw the correct Lewis structure for the molecule.
- Then, the electron domain around the central atom should be counted and the geometry that matches with that type of domain in VSEPR should be determined.
- Finally, the geometry is predicted by using the orientation of atoms.
The molecules with considering the domains of type
Lewis structure for any molecule is drawn by using the following steps,
First the skeletal structure for the given molecule is drawn then the total number of valence electrons for all atoms present in the molecule is determined
The next step is to subtract the electrons present in the total number of bonds present in the skeletal structure of the molecule with the total valence electrons such that considering each bond contains two electrons with it.
Finally, the electrons which got after subtractions has to be equally distributed such that each atom contains eight electrons in its valence shell.
Electron Domain: In VSEPR theory, both the lone pair and the bonded pair are together considered as electron domain regardless of the type of bond in which the bonded pair presents.
(e)
Answer to Problem 7.6QP
(e)
Tetrahedral
Explanation of Solution
To predict: The geometry for the given molecule.
Draw the Lewis structure for the molecule (e)
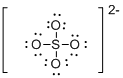
First the skeletal structure for the given molecule is drawn then the total number of valence electrons in the molecule is 30.
The next step is to subtract the electrons present in the total number of bonds present in the molecule with the total valence electrons such that 8 has to be subtracted with 30 as each bond contains two electrons with it and there are four bonds in the skeletal structure.
Finally, the 22 electrons got after subtractions plus the two electrons due to the charge -2 has to be equally distributed over oxygen atoms such that each atom contains eight electrons in its valence shell.
Determine the molecular geometry for the molecule (e) using VSEPR.
The electron domain for the given molecule is obtained by viewing the Lewis structure which is of type tetrahedral since there are four atoms around the central atom and the molecular geometry is also tetrahedral due to the absence of lone pair over the central atom
Want to see more full solutions like this?
Chapter 7 Solutions
Chemistry: Atoms First
- Please correct answer and don't used hand raitingarrow_forward(11pts total) Consider the arrows pointing at three different carbon-carbon bonds in the molecule depicted below. Bond B Bond A Bond C a. (2pts) Which bond between A-C is weakest? Which is strongest? Place answers in appropriate boxes. Weakest Bond Strongest Bond b. (4pts) Consider the relative stability of all cleavage products that form when bonds A, B, AND C are homolytically cleaved/broken. Hint: cleavage products of bonds A, B, and C are all carbon radicals. i. Which ONE cleavage product is the most stable? A condensed or bond line representation is fine. ii. Which ONE cleavage product is the least stable? A condensed or bond line representation is fine. c. (5pts) Use principles discussed in lecture, supported by relevant structures, to succinctly explain the why your part b (i) radical is more stable than your part b(ii) radical. Written explanation can be no more than one-two succinct sentence(s)!arrow_forward. 3°C with TH 12. (10pts total) Provide the major product for each reaction depicted below. If no reaction occurs write NR. Assume heat dissipation is carefully controlled in the fluorine reaction. 3H 24 total (30) 24 21 2h • 6H total ● 8H total 34 래 Br2 hv major product will be most Substituted 12 hv Br NR I too weak of a participate in P-1 F₂ hv Statistically most favored product will be major = most subst = thermo favored hydrogen atom abstractor to LL Farrow_forward
- Five chemistry project topic that does not involve practicalarrow_forwardPlease correct answer and don't used hand raitingarrow_forwardQ2. Consider the hydrogenation of ethylene C2H4 + H2 = C2H6 The heats of combustion and molar entropies for the three gases at 298 K are given by: C2H4 C2H6 H2 AH comb/kJ mol¹ -1395 -1550 -243 Sº / J K¹ mol-1 220.7 230.4 131.1 The average heat capacity change, ACP, for the reaction over the temperature range 298-1000 K is 10.9 J K¹ mol¹. Using these data, determine: (a) the standard enthalpy change at 800 K (b) the standard entropy change at 800 K (c) the equilibrium constant at 800 K.arrow_forward
- 13. (11pts total) Consider the arrows pointing at three different carbon-carbon bonds in the molecule depicted below. Bond B Bond A Bond C a. (2pts) Which bond between A-C is weakest? Which is strongest? Place answers in appropriate boxes. Weakest Bond Strongest Bond b. (4pts) Consider the relative stability of all cleavage products that form when bonds A, B, AND C are homolytically cleaved/broken. Hint: cleavage products of bonds A, B, and C are all carbon radicals. i. Which ONE cleavage product is the most stable? A condensed or bond line representation is fine. ii. Which ONE cleavage product is the least stable? A condensed or bond line representation is fine. c. (5pts) Use principles discussed in lecture, supported by relevant structures, to succinctly explain the why your part b (i) radical is more stable than your part b(ii) radical. Written explanation can be no more than one-two succinct sentence(s)! Googlearrow_forwardPrint Last Name, First Name Initial Statifically more chances to abstract one of these 6H 11. (10pts total) Consider the radical chlorination of 1,3-diethylcyclohexane depicted below. 4 4th total • 6H total 래 • 4H total 21 total ZH 2H Statistical H < 3° C-H weakest - product abstraction here bund leads to thermo favored a) (6pts) How many unique mono-chlorinated products can be formed and what are the structures for the thermodynamically and statistically favored products? Product 6 Number of Unique Mono-Chlorinated Products Thermodynamically Favored Product Statistically Favored Product b) (4pts) Draw the arrow pushing mechanism for the FIRST propagation step (p-1) for the formation of the thermodynamically favored product. Only draw the p-1 step. You do not need to include lone pairs of electrons. No enthalpy calculation necessary H H-Cl Waterfoxarrow_forward10. (5pts) Provide the complete arrow pushing mechanism for the chemical transformation → depicted below Use proper curved arrow notation that explicitly illustrates all bonds being broken, and all bonds formed in the transformation. Also, be sure to include all lone pairs and formal charges on all atoms involved in the flow of electrons. CH3O II HA H CH3O-H H ①arrow_forward
- Do the Lone Pairs get added bc its valence e's are a total of 6 for oxygen and that completes it or due to other reasons. How do we know the particular indication of such.arrow_forwardNGLISH b) Identify the bonds present in the molecule drawn (s) above. (break) State the function of the following equipments found in laboratory. Omka) a) Gas mask b) Fire extinguisher c) Safety glasses 4. 60cm³ of oxygen gas diffused through a porous hole in 50 seconds. How long w 80cm³ of sulphur(IV) oxide to diffuse through the same hole under the same conditions (S-32.0.0-16.0) (3 m 5. In an experiment, a piece of magnesium ribbon was cleaned with steel w clean magnesium ribbon was placed in a crucible and completely burnt in oxy cooling the product weighed 4.0g a) Explain why it is necessary to clean magnesium ribbon. Masterclass Holiday assignmen PB 2arrow_forwardHi!! Please provide a solution that is handwritten. Ensure all figures, reaction mechanisms (with arrows and lone pairs please!!), and structures are clearly drawn to illustrate the synthesis of the product as per the standards of a third year organic chemistry course. ****the solution must include all steps, mechanisms, and intermediate structures as required. Please hand-draw the mechanisms and structures to support your explanation. Don’t give me AI-generated diagrams or text-based explanations, no wordy explanations on how to draw the structures I need help with the exact mechanism hand drawn by you!!! I am reposting this—ensure all parts of the question are straightforward and clear or please let another expert handle it thanks!!arrow_forward
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning





