
Concept explainers
What is the major product obtained from the reaction of each of the following compounds with excess HCl?
- a. CH3CH2C≡CH
- b. CH3CH2C≡CCH2CH3
- c. CH3CH2C≡CCH2CH2CH3
(a)
Interpretation:
The major product obtained from the reaction of given compound with excess of
Concept Introduction:
Addition of halides to alkynes: Halogens like chlorine and bromine adds to the alkyne compounds. The general form of the reaction is given below.
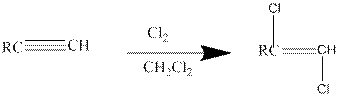
If excess halides are present, a second addition reaction also occurs.
Answer to Problem 27P
The major product obtained from the reaction of given compound with excess of

Explanation of Solution
Hydrogen halides undergo addition reaction with alkynes. Alkyne is a nucleophile and it reacts with the electrophile to give a

In this reaction of butyne with
(b)
Interpretation:
The major product obtained from the reaction of given compound with excess of
Concept Introduction:
Addition of halides to alkynes: Halogens like chlorine and bromine adds to the alkyne compounds. The general form of the reaction is given below.
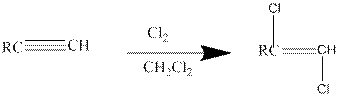
If excess halides are present, a second addition reaction also occurs.
Answer to Problem 27P
The major product obtained from the reaction of given compound with excess of

Explanation of Solution
Hydrogen halides undergo addition reaction with alkynes. Alkyne is a nucleophile and it reacts with the electrophile to give a

In this reaction of hex-3-yne with
(c)
Interpretation:
The major product obtained from the reaction of given compound with excess of
Concept Introduction:
Addition of halides to alkynes: Halogens like chlorine and bromine adds to the alkyne compounds. The general form of the reaction is given below.
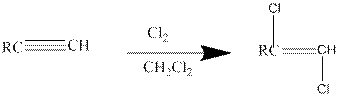
If excess halides are present, a second addition reaction also occurs.
Answer to Problem 27P
The major product obtained from the reaction of given compound with excess of
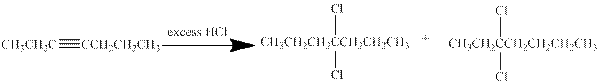
Explanation of Solution
Hydrogen halides undergo addition reaction with alkynes. Alkyne is a nucleophile and it reacts with the electrophile to give a
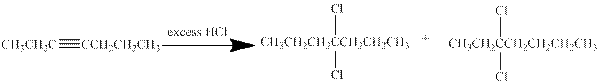
In this reaction of hept-3-yne with
Want to see more full solutions like this?
Chapter 7 Solutions
EBK ORGANIC CHEMISTRY
- You're competing on a Great British television game show, and you need to bake a cake. The quantity for each ingredient is given in grams, but you haven't been given a kitchen scale. Which of these properties would correlate with the mass of a baking ingredient like eggs or milk? Check all that apply. depth of color viscosity volume densityarrow_forwardDraw a Lewis structure for each of the following species. Again, assign charges where appropriate. a. H-H¯ b. CH3-CH3 c. CH3+CH3 d. CH3 CH3 e. CH3NH3+CH3NH3 f. CH30-CH3O¯ g. CH2CH2 - h. HC2-(HCC) HC2 (HCC) i. H202×(HOOH) H₂O₂ (HOOH) Nortonarrow_forwardIs molecule 6 an enantiomer?arrow_forward
- Show work. Don't give Ai generated solutionarrow_forwardCheck the box under each structure in the table that is an enantiomer of the molecule shown below. If none of them are, check the none of the above box under the table. Molecule 1 Molecule 2 Molecule 3 ----||| Molecule 4 Molecule 5 Molecule 6 none of the above mm..arrow_forwardShow work. don't give Ai generated solutionarrow_forward
- Check the box under each structure in the table that is an enantiomer of the molecule shown below. If none of them are, check the none of the above box under the table. Molecule 1 Molecule 2 Molecule 3 ----||| Molecule 4 Molecule 5 Molecule 6 none of the above mm..arrow_forwardUse the vapor-liquid equilibrium data at 1.0 atm. for methanol-water (Table 2-8 ) for the following: If the methanol vapor mole fraction is 0.600, what is the methanol liquid mole fraction? Is there an azeotrope in the methanol-water system at a pressure of 1.0 atmospheres? If water liquid mole fraction is 0.350, what is the water vapor mole fraction? What are the K values of methanol and of water at a methanol mole fraction in the liquid of 0.200? What is the relative volatility αM-W at a methanol mole fraction in the liquid of 0.200?arrow_forwardCheck the box under each structure in the table that is an enantiomer of the molecule shown below. If none of them are, check the none of the above box under the table. || |II***** Molecule 1 | Molecule 4 none of the above Molecule 2 Molecule 3 Х mm... C ---||| *** Molecule 5 Molecule 6arrow_forward
- is SiBr4 Silicon (IV) tetra Bromine? is KClO2 potassium dihypochlorite ?arrow_forward"יוון HO" Br CI Check the box under each structure in the table that is an enantiomer of the molecule shown below. If none of them are, check the none of the above box under the table. Molecule 1 Molecule 2 Molecule 3 Br Br Br HO OH H CI OH ✓ Molecule 4 Molecule 5 Molecule 6 CI Br יייון H Br OH OH CI Br ☐ none of the above × Garrow_forwardUS2 Would this be Uranium (II) diSulfide?arrow_forward
