
Concept explainers
(a)
Interpretation:
An oxygen-binding curve for a hypothetical two subunit hemoglobin with
Concept introduction:
Hill equation is represented as follows:
Here, Y- fractional saturation
n − a measure of the degree of cooperativity in ligand binding.
P50 − partial pressure of oxygen at which hemoglobin is half saturated.
The Hill plot of log(Y/1-Y) versus log(P50) should be a linear graph with slope of n.
Answer to Problem 14P
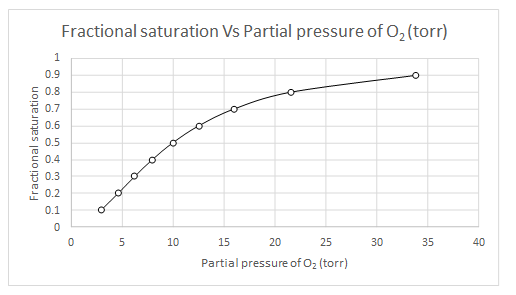
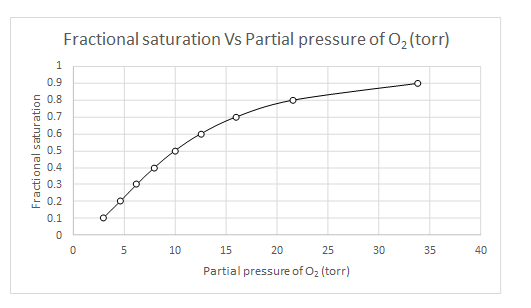
Explanation of Solution
The values of
| Y | log(Y/(1-Y) | log(Y/(1-Y)-n log (P50) | log (pO2) | pO2 |
| 0.1 | -0.95424251 | 0.845757491 | 0.469865 | 2.950294 |
| 0.2 | -0.60205999 | 1.197940009 | 0.665522 | 4.629374 |
| 0.3 | -0.36797679 | 1.432023215 | 0.795568 | 6.245518 |
| 0.4 | -0.17609126 | 1.623908741 | 0.902172 | 7.983099 |
| 0.5 | 0 | 1.8 | 1 | 10 |
| 0.6 | 0.176091259 | 1.976091259 | 1.097828 | 12.52646 |
| 0.7 | 0.367976785 | 2.167976785 | 1.204432 | 16.01148 |
| 0.8 | 0.602059991 | 2.402059991 | 1.334478 | 21.60119 |
| 0.9 | 0.954242509 | 2.754242509 | 1.530135 | 33.89493 |
(b)
Interpretation:
An oxygen-binding curve for shypothetical two subunit hemoglobin with
Concept introduction:
Concerted model equation is written as follows:
Here, Y − fractional saturation
a − ratio between the substrate concentration and the dissociation constant for a ligand binding to a single site in R state.
L − The ratio of the concentrations of the T and R states with no ligands bound
c − the ratio between the dissociation constant for a ligand binding to a single site in R state and that of T state.
n − number of binding sites
Also, the ratio can be calculated as follows:
Here,
pO2 − partial pressure of oxygen
KR - the dissociation constant for a ligand binding to a single site in R state.
Answer to Problem 14P
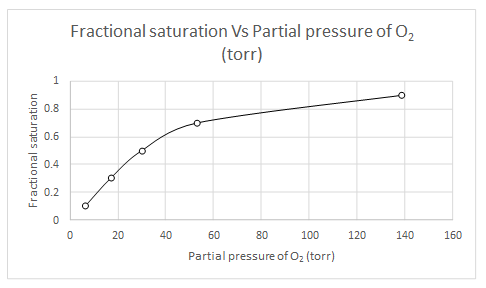
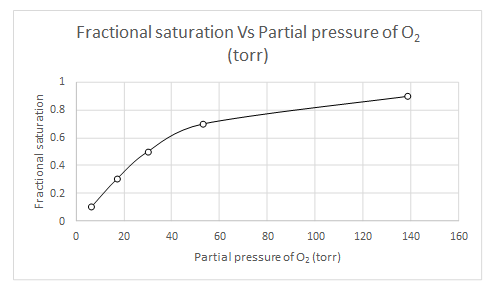
Explanation of Solution
When
Likewise, partial pressure of oxygen for several fractional saturation values are calculated and a plot of fractional saturation versus oxygen partial pressure is drawn.
| Y | pO2 |
| 0.1 | 6.55 |
| 0.3 | 17.09 |
| 0.5 | 30.15 |
| 0.7 | 53.21 |
| 0.9 | 138.91 |
Want to see more full solutions like this?
Chapter 7 Solutions
BIOCHEMISTRY (LOOSELEAF)-W/ACCESS
- TABLE 3-LACTATE PRODUCTION IN FORTIFIED HEMOLYSATES OF HUMAN ERYTHROCYTES* Substrate Glucose Glucose Lactate production† No. of experiments pH 6 7.1 2.03 ± 0.91 6 7.8 4.76 ± 1.09 7-1 10-73 +1-88 5 7.8 12.34 ±2.92 5 7.0 7-15±0.73 5 7-7 (b)( ) In mature erythrocytes (red blood cells) the end product of glycolysis is lactate because of the absence of mitochondria. On the right is a table comparing the rate of lac- tate production in hemolysates (lysed cells) of human RBCs as a function of pH with dif- ferent substrates introduced into the glyco- lytic pathway. The hemolysate was fortified with 30 μmoles substrate, 7.5 μmoles MgCl2, 10 μmoles disodium phosphate, 1.5 μmoles NAD and 5 μmoles ATP in a volume of 5 mL. The rate of lactate production is given as μmoles of lactate/g Hb/hr at 37° C, buffered to either pH 7.1 or 7.8, as indicated. According to the results in the table which glycolytic enzyme is rate-limiting? Explain. Glucose-6-phosphate Glucose-6-phosphate Fructose-1,6-diphosphate…arrow_forwardPrevious Assignment: The figure below shows the bonding of the cytosine and guanine molecules. The O - H and H - N distances are each 0.110 nm. In this case, assume that the bonding is due only to the forces along the O - H - O, N - H - N and O - H - N combinations, and assume also that these three combinations are parallel to each other. Calculate the net force that cytosine exerts on guanine due to the preceding three combinations. Is this force attractive or repulsive? ---------------------------------------------------------------------------------- Electric Potential Energy: Base Pairing in DNA, IICalculate the electric potential energy of the guanine–cytosine bond, using the same combinations of molecules (O - H - O, N - H - N and O - H - N) as in the previous assignment (pls refer above)arrow_forwardIn a molecular disease of hemoglobin, Hemoglobin Rainier, Tyr 145β is replaced by Cys, which forms a disulfide bond with another Cysresidue in the same subunit. This prevents the formation of ion pairs that normally stabilize the T state. How does hemoglobin Rainier differ from normal hemoglobin with respect to (A)oxygen affinity, (B)the Bohr effect, and (C)the Hillcoefficient? Explain your answers.arrow_forward
- Que:- An Michaels - Menten Kenetirs eneyme obeying the following parameters K2= 1•4 x10%M's" , K,= 2:5x1o°5' gave - 1 K3 = l-5 x10s ca) So this enzyme ctalytically pufet ? Explain your heasoning . (b) What is Vmaz , ik 1-4 nmol/me and saturabing eveyrme are usecd ? substratearrow_forwardq41 please calculate the unknown concentration of the protein D wih an absorbance value of A412 given the standard curve indicated in the table. write your answers in numbers only with 2 decimals. protein concentration (ug/ml) absorbance 0 0.000 0.02 0.161 0.04 0.284 0.06 0.438 0.08 0.572 0.10 0.762arrow_forwardA variant of hemoglobin (Boston variant; mutation His E7(58)α → Tyr) promotes methemoglobin formation involving the α (alpha) subunits. What is the maximum value of the Hill constant (n) that you could measure for the Boston variant of hemoglobin? log (YO2 / 1 - YO2 ) = log pO2 - logP50 Please break down each step of the Hill equation and explain why the result for n is valid from a logical standpoint.arrow_forward
- Many enzymes obey simple Michaelis–Mentenkinetics, which are summarized by the equationrate = Vmax [S]/([S] + Km)where Vmax = maximum velocity, [S] = concentration ofsubstrate, and Km = the Michaelis constant.It is instructive to plug a few values of [S] into theequation to see how rate is affected. What are the rates for[S] equal to zero, equal to Km, and equal to infinite concen-tration?arrow_forwardNonspecific elution of affinity bonded macromolecules is used in affinity chromatography explain why?arrow_forwardCalculate the actual free energy of hydrolysis of ATP, delta Gp in the erythrocytes of a new species. The standard free-energy of hydrolysis of ATP is also -30.5kJ/mol in this species, but the concentrations in this specie's erythrocytes are 0.00760 mM ATP, 0.00063 mM ADP and 0.00273 mM Pi. Assume the pH is 7.0 and the body temperature of this species is 91.0oC. Calculate your answer as kJ/mol to two decimal places.arrow_forward
- Protein purification table: A 50 ml crude skeletal muscle extract contains 32mg of protein per ml. Ten ul of the extract catalyzes a reaction at a rate of 0.14 µmol product per minute. The extract was fractionated by ammonium sulfate precipitation, and the fraction precipitating between 20% and 40% saturation was dissolved in 10ml. The solution contains 50mg/ml protein. Ten ul of this purified fraction catalyzes the reaction at a rate of 0.65 µmol/min (a) What is the degree of purification (fold purification)? (b) What is the percent yield of the enzyme recovered in the purification?arrow_forward"The native structure of hemoglobin (HB) comprises of two α and two β subunits, each of which carries a heme group. There appear to be no previous studies that report the in-vitro folding and assembly of Hb from highly unfolded α and β globin in a 'one-pot' reaction. One difficulty that has to be overcome for studies of this kind is the tendency of Hb to aggregate during refolding. This work demonstrates that denaturation of Hb in 40% acetonitrile at pH 10.0 is reversible." (J Am Soc Mass Spectrum 2007, 18, 8-16)Based on the information in the passage, the total number of heme groups present in four hemoglobin protein molecules is ________. a. 16 b. 4 c. 12 d. 8arrow_forward"The native structure of hemoglobin (Hb) comprises of two α and two β subunits, each of which carries a heme group. There appear to be no previous studies that report the in-vitro folding and assembly of Hb from highly unfolded α and β globin in a 'one-pot' reaction. One difficulty that has to be overcome for studies of this kind is the tendency of Hb to aggregate during refolding. This work demonstrates that denaturation of Hb in 40% acetonitrile at pH 10.0 is reversible." (J Am Soc Mass Spectrum 2007, 18, 8-16)Hemoglobin is ________.arrow_forward
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON





