
Biology (MindTap Course List)
11th Edition
ISBN: 9781337392938
Author: Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. Berg
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 7, Problem 11TYU
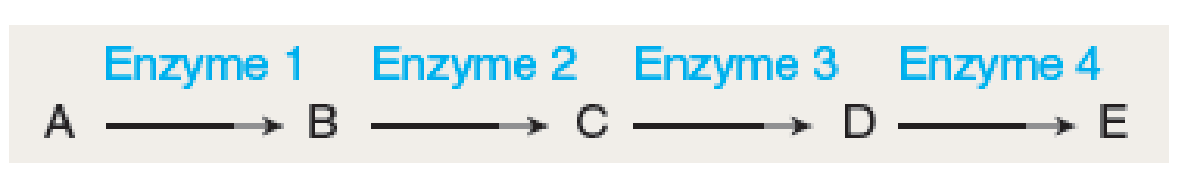
PREDICT In the following reaction series, which enzyme(s) is/are most likely to have an allosteric site to which the end product E binds? (a) enzyme 1 (b) enzyme 2 (c) enzyme 3 (d) enzyme 4 (e) enzymes 3 and 4
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
A substrate is converted to a product via the reaction sequence
(1) E +S ES
k2
(2) ES + S ES2
k4
(3) ES2
ES +P
(4) ES , E +P
(a). Using pseudo-steady state hypothesis for various forms of enzyme active sites, obtain relations
between rates of individual steps in the mechanism.
(b). Relating concentrations of vacant active sites to concentrations of occupied active sites, obtain
expressions for the rate of consumption of S, (-Rs), and the rate of formation of P, Rp.
(c). What are the maximum values of (-Rs) and Rp and under which conditions are these attained?
(a)
The Michaelis-Menten equation was derived for the "hypothetical" reaction written
below involving only one intermediate:
E + S
ES
E
+
P
[1]
A generalized representation of an enzyme catalyzed reaction that is likely to be more accurate and
closer to reality can be written as follows:
E + S
P [2]
E +
¿
where the arrows in the forward and backward directions indicate at least partial reversibility for each
step, 12 and 13 represent reaction intermediates sequentially formed after the noncovalent Michaelis
complex ES, and EP indicates an enzyme-product complex. Nonetheless, the Michaelis equation
ES
12
13
EP
V
Kcat [Eo] [SO] (KM + [SO])
can be used to determine kinetic parameters under steady-state conditions. Explain.
(b)
Determination of kinetic parameters governing an enzyme catalyzed reaction under
steady-state conditions leads to estimation of values for kcat, KM, and kcat/KM. Define each parameter
and indicate which are determined according to the quality of the experimental…
The following reaction coordinate diagram charts the energy of a substrate molecule (S) as it passes through a transition state (X‡) on its way to becoming a stable product (P) alone or in the presence of one of two different enzymes (E1 and E2). How does the addition of either enzyme affect the change in Gibbs free energy (ΔG) for the reaction? Which of the two enzymes binds with greater affinity to the substrate? Which enzyme better stabilizes the transition state? Which enzyme functions as a better catalyst?
Chapter 7 Solutions
Biology (MindTap Course List)
Ch. 7.1 - Define energy, emphasizing how it is related to...Ch. 7.1 - Use examples to contrast potential energy and...Ch. 7.1 - Prob. 1CCh. 7.2 - Prob. 3LOCh. 7.2 - Prob. 1CCh. 7.2 - Life is sometimes described as a constant struggle...Ch. 7.3 - Prob. 4LOCh. 7.3 - Prob. 5LOCh. 7.3 - Prob. 6LOCh. 7.3 - Prob. 1C
Ch. 7.3 - Prob. 2CCh. 7.4 - Explain how the chemical structure of ATP allows...Ch. 7.4 - Prob. 1CCh. 7.4 - Prob. 2CCh. 7.5 - Relate the transfer of electrons (or hydrogen...Ch. 7.5 - PREDICT Which has the most energy, the oxidized...Ch. 7.6 - Explain how an enzyme lowers the required energy...Ch. 7.6 - Describe specific ways enzymes are regulated.Ch. 7.6 - Prob. 1CCh. 7.6 - How does the function of the active site of an...Ch. 7.6 - How are temperature and pH optima of an enzyme...Ch. 7.6 - Prob. 4CCh. 7 - Which of the following can do work in a cell? (a)...Ch. 7 - Prob. 2TYUCh. 7 - Prob. 3TYUCh. 7 - Test Your Understanding 4. Diffusion is an (a)...Ch. 7 - Prob. 5TYUCh. 7 - Prob. 6TYUCh. 7 - Prob. 7TYUCh. 7 - Test Your Understanding 8. Induced fit means that...Ch. 7 - Prob. 9TYUCh. 7 - Prob. 10TYUCh. 7 - PREDICT In the following reaction series, which...Ch. 7 - Test Your Understanding 12. EVOLUTION link All...Ch. 7 - EVOLUTION LINK Some have argued that evolution is...Ch. 7 - Prob. 14TYUCh. 7 - Prob. 15TYUCh. 7 - Prob. 16TYU
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Similar questions
- Rearrange the following terms to show the process of enzymatic reaction. Use and + to complete the equation. enzyme enzyme-substrate complex enzyme product substrate Several important things should be noted about this reaction: 1. A/an because of the fit between their structures; 2. As a result, something happens to the example, it might be split in two at a particular location. 3. Then the and 4. The enzyme is again. 5. Note that the arrows in the formula for enzyme reaction point acts on a specific to form a/an molecule. For comes apart, yielding the in the reaction and is now free to react _- This means that the reaction is 6. An enzyme-substrate complex can simply go back to the the 7. The products of an enzymatic reaction can react with the enzyme to form the and again; 8. It, in turn, may again form the 9. Therefore, the same. and the may act to cause a to go either way.arrow_forwardIn enzyme and chemical kinetics A -->P, what is the instantaneous appearance or disappearance of A called?: 1) Velocity v = d[E]/dt 2) Velocity v = -d[A]/dt 3) Velocity v = d[P]/dt 4) 2&3 Can be correct.arrow_forwardThe total concentration of enzyme in a reaction, [E], is made up of the concentration of enzyme bound to substrate, [ES], and the concentration of enzyme still free in solution, [Ef]. Similarly, the total amount of substrate is made up of [Sf] and [ES]. We can assume that the concentration of enzyme is much less than that of the substrate, [E] << [S] Explain why [S] >> [ES] Hence explain why [Sf] ~ [S]arrow_forward
- (a) If the total enzyme concentration 1 nmol/L, how many molecules of substrate can a molecule of enzyme process in each minute?(b) Calculate kcat/KM for the enzyme reaction. Is this a fairly efficient enzyme? (as shown.)arrow_forwardYou are working on an enzyme that obeys standard Michaelis-Menten kinetics. What variable is the V, dependant on if the concentration of the substrate is substantially higher than the concentration of the enzyme? [S] [E] [ES] O [P] O not enough information providedarrow_forwardEnzyme X and enzyme Y catalyze the same reaction and exhibit the νo versus [S] curves shown below. Which enzyme is more effi cient at low [S]? Which is more effi cient at high [S]?arrow_forward
- How many of the following statements are true? Allosteric enzymes display sigmoidal kinetics for plots of V versus [S] Allosteric enzymes exist in T and R states Allosteric enzymes display kinetics that becomes less sigmoidal in the presence of activatorsarrow_forwardThe total concentration of enzyme in a reaction, [E], is made up of the concentration of enzyme bound to substrate, [ES], and the concentration of enzyme still free in solution, [Ef]. Similarly, the total amount of substrate is made up of [Sf] and [ES]. We can assume that the concentration of enzyme is much less than that of the substrate, [E] << [S]. Assuming the steady state condition and the relationships between [E], [Ef] and [ES], and similar ones for S, given in lectures, derive an expression for the saturation factor, , in terms of [S] and . (Note that [E] and [S] denote the total amounts of enzyme and substrate added to the reaction, respectively. You may assume that [S]>>[E].)arrow_forwardConsider two enzymes catalyzing two reactions (A --> B --> C ) in a metabolic cascade with their properties summarized below: Keg (for reaction) Enzyme Reaction KM Kcat / KM 102 M-1s-1 108 M-1s-1 1 A --> B 1 1 mM 2 B --> C 10 10 mM Initial concentrations are [A] = 0.1 mM and [B] = [C] = 0 and both enzymes are present at concentrations of 1 mM. After waiting for 1 ms, the concentrations of A, B, and C are measured. How do you expect the concentrations to be ordered? O [B] > [A] > [C] O [C] > [B] > [A] O [A] > [B] > [C] O [A] > [C] > [B] [C] > [A] > [B]arrow_forward
- You have obtained experimental kinetic data for two versions of the same enzyme, a wild‑type and a mutant differing from the wild‑type at a single amino acid. The data are given in the table. Compare the kinetic parameters of the two versions using the data in the table. Assuming a two-step reaction scheme in which ?−1 is much larger than ?2, which of the following statements are correct? The mutant version has a higher affinity for the substrate. The wild‑type version requires a greater concentration of substrate to achieve ?maxVmax. The wild‑type version has a higher affinity for the substrate. The mutant version requires a greater concentration of substrate to achieve ?maxVmax. Calculate the initial velocity of the reaction catalyzed by the wild‑type enzyme when the substrate concentration is 10 mM. The reaction equilibrium is reached once there is no net change in the concentration of the substrate or the product. Based on the data table and your initial…arrow_forwardWhich of the following statements about isozymes is correct? (Select all that apply.) (a) Isozymes allow reactions to be optimized under different conditions. (b) The main reason for isozymes is so that a given reaction is never completely inhibited. (c) Isozymes have completely different active sites. (d) Bind the same substrates but form different products. (e) Isozymes display different physical properties, such as kinetic parameters.arrow_forwardWhat general effects would you expect the following changes to have on the rate of an enzyme-catalyzed reaction for an enzyme that has its maximum activity at body temperature (about 37 °C/310.15 K)?(a) Raising the temperature from 310 K (37 °C) to 343 K (70 °C)(b) Lowering the pH from 7 to 3(c) Adding an organic solvent, such as methanolarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON
Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education,
Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education, Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company
Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co.
Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co. Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education
Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education

Human Anatomy & Physiology (11th Edition)
Biology
ISBN:9780134580999
Author:Elaine N. Marieb, Katja N. Hoehn
Publisher:PEARSON

Biology 2e
Biology
ISBN:9781947172517
Author:Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:OpenStax

Anatomy & Physiology
Biology
ISBN:9781259398629
Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa Stouter
Publisher:Mcgraw Hill Education,

Molecular Biology of the Cell (Sixth Edition)
Biology
ISBN:9780815344322
Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter Walter
Publisher:W. W. Norton & Company

Laboratory Manual For Human Anatomy & Physiology
Biology
ISBN:9781260159363
Author:Martin, Terry R., Prentice-craver, Cynthia
Publisher:McGraw-Hill Publishing Co.

Inquiry Into Life (16th Edition)
Biology
ISBN:9781260231700
Author:Sylvia S. Mader, Michael Windelspecht
Publisher:McGraw Hill Education
Enzyme Kinetics; Author: MIT OpenCourseWare;https://www.youtube.com/watch?v=FXWZr3mscUo;License: Standard Youtube License