
Organic Chemistry
5th Edition
ISBN: 9780078021558
Author: Janice Gorzynski Smith Dr.
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 6, Problem 6.12P
The equilibrium constant for the conversion of the axial to the equatorial conformation of methoxycyclohexane is
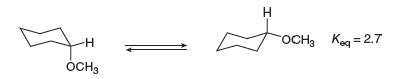
a. Given these data, which conformation is present in the larger amount at equilibrium?
b. Is
c. From the values in Table
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Don't used Ai solution
Don't used Ai solution
Please correct answer and don't used hand raiting
Chapter 6 Solutions
Organic Chemistry
Ch. 6 - Problem 6.1 Classify each transformation as...Ch. 6 - Prob. 6.2PCh. 6 - Problem 6.3 By taking into account...Ch. 6 - Problem 6.4 Use curved arrows to show the movement...Ch. 6 - Problem 6.5 Follow the curved arrows and draw the...Ch. 6 - Prob. 6.6PCh. 6 - Problem 6.7 Use the values in Table 6.2 to...Ch. 6 - Prob. 6.8PCh. 6 - aWhich Keq corresponds to a negative value of G,...Ch. 6 - Given each of the following values, is the...
Ch. 6 - Given each of the following values, is the...Ch. 6 - The equilibrium constant for the conversion of the...Ch. 6 - Prob. 6.13PCh. 6 - For a reaction with H=40kJ/mol, decide which of...Ch. 6 - For a reaction with H=20kJ/mol, decide which of...Ch. 6 - Draw an energy diagram for a reaction in which the...Ch. 6 - Prob. 6.17PCh. 6 - Prob. 6.18PCh. 6 - Problem 6.19 Consider the following energy...Ch. 6 - Draw an energy diagram for a two-step reaction,...Ch. 6 - Which value if any corresponds to a faster...Ch. 6 - Prob. 6.22PCh. 6 - Problem 6.23 For each rate equation, what effect...Ch. 6 - Prob. 6.24PCh. 6 - Identify the catalyst in each equation. a....Ch. 6 - Draw the products of homolysis or heterolysis of...Ch. 6 - Explain why the bond dissociation energy for bond...Ch. 6 - Classify each transformation as substitution,...Ch. 6 - Prob. 6.29PCh. 6 - 6.30 Draw the products of each reaction by...Ch. 6 - 6.31 (a) Add curved arrows for each step to show...Ch. 6 - Prob. 6.32PCh. 6 - Prob. 6.33PCh. 6 - Prob. 6.34PCh. 6 - Calculate H for each reaction. a HO+CH4CH3+H2O b...Ch. 6 - Homolysis of the indicated CH bond in propene...Ch. 6 - Prob. 6.37PCh. 6 - Prob. 6.38PCh. 6 - 6.39. a. Which value corresponds to a negative...Ch. 6 - Prob. 6.40PCh. 6 - For which of the following reaction is S a...Ch. 6 - Prob. 6.42PCh. 6 - Prob. 6.43PCh. 6 - 6.44 Consider the following reaction: .
Use curved...Ch. 6 - Prob. 6.45PCh. 6 - Draw an energy diagram for the Bronsted-Lowry...Ch. 6 - Prob. 6.47PCh. 6 - Indicate which factors affect the rate of a...Ch. 6 - Prob. 6.49PCh. 6 - 6.50 The conversion of acetyl chloride to methyl...Ch. 6 - Prob. 6.51PCh. 6 - Prob. 6.52PCh. 6 - The conversion of (CH3)3Cl to (CH3)2C=CH2 can...Ch. 6 - 6.54 Explain why is more acidic than , even...Ch. 6 - Prob. 6.55PCh. 6 - Prob. 6.56PCh. 6 - Prob. 6.57PCh. 6 - Although Keq of equation 1 in problem 6.57 does...Ch. 6 - Prob. 6.59P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- (12) Which one of the following statements about fluo- rometry is FALSE? a) Fluorescence is better detected at 90 from the exci- tation direction. b) Fluorescence is typically shifted to longer wave- length from the excitation wavelength. c) For most fluorescent compounds, radiation is pro- duced by a transitionarrow_forwardDon't used Ai solutionarrow_forwardDon't used Ai solutionarrow_forward
- Don't used Ai solutionarrow_forwardIndicate the correct option.a) Graphite conducts electricity, being an isotropic materialb) Graphite is not a conductor of electricityc) Both are falsearrow_forward(f) SO: Best Lewis Structure 3 e group geometry:_ shape/molecular geometry:, (g) CF2CF2 Best Lewis Structure polarity: e group arrangement:_ shape/molecular geometry: (h) (NH4)2SO4 Best Lewis Structure polarity: e group arrangement: shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles): Sketch (with angles):arrow_forward
- 1. Problem Set 3b Chem 141 For each of the following compounds draw the BEST Lewis Structure then sketch the molecule (showing bond angles). Identify (i) electron group geometry (ii) shape around EACH central atom (iii) whether the molecule is polar or non-polar (iv) (a) SeF4 Best Lewis Structure e group arrangement:_ shape/molecular geometry: polarity: (b) AsOBr3 Best Lewis Structure e group arrangement:_ shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles):arrow_forward(c) SOCI Best Lewis Structure 2 e group arrangement: shape/molecular geometry:_ (d) PCls Best Lewis Structure polarity: e group geometry:_ shape/molecular geometry:_ (e) Ba(BrO2): Best Lewis Structure polarity: e group arrangement: shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles): Sketch (with angles):arrow_forwardDon't used Ai solutionarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
The Laws of Thermodynamics, Entropy, and Gibbs Free Energy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=8N1BxHgsoOw;License: Standard YouTube License, CC-BY