
Organic Chemistry - Standalone book
10th Edition
ISBN: 9780073511214
Author: Francis A Carey Dr., Robert M. Giuliano
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 6, Problem 30P
The reaction of cyclopentyl bromide with sodium cyanide to give cyclopentyl cyanide
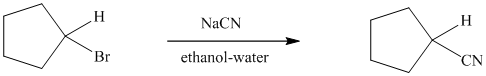
proceeds faster if a small amount of sodium iodide is added to the reaction mixture. Can you suggest a reasonable mechanism to explain the catalytic function of sodium iodide?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
If the rate of reaction of [0.1 M] sodium cyanide with [0.1 M] 2-bromo-2-methylpropane is 1.2 mole/second, what would be the effect on theoverall rate if the concentration of sodium cyanide is increased to [0.2 M] and the concentration of the alkyl bromide is decreased to [0.05 M]?
Write the mechanism, as well as the structure of intermediate A and product B, for the following reaction.
Write a mechanism that explains how the reaction of 1 mol of bromoethane with 1 mol of ammonia can lead to a mixture ethylamine,diethylamine,triethylamine and tetraethylammonium bromite rather than pure ethylamine
Chapter 6 Solutions
Organic Chemistry - Standalone book
Ch. 6.1 - Prob. 1PCh. 6.2 - 1-Bromo-3-chloropropane reacts with one molar...Ch. 6.3 - Prob. 3PCh. 6.3 - The Fischer projection for (+)-2-bromooctane is...Ch. 6.3 - Would you expect the 2-octanol formed by SN2...Ch. 6.3 - Prob. 6PCh. 6.4 - Prob. 7PCh. 6.4 - The first step in the synthesis of the...Ch. 6.6 - Prob. 9PCh. 6.6 - Prob. 10P
Ch. 6.7 - Prob. 11PCh. 6.8 - Prob. 12PCh. 6.9 - Diethyl ether (CH3CH2OCH2CH3) has a dielectric...Ch. 6.9 - Unlike protic solvent which solvate from complexes...Ch. 6.10 - Prob. 15PCh. 6.10 - Prob. 16PCh. 6.10 - The hydrolysis of sulfonate of 2-octanol is...Ch. 6.11 - Prob. 18PCh. 6 - Prob. 19PCh. 6 - Prob. 20PCh. 6 - Both of the following reactions involve...Ch. 6 - Prob. 22PCh. 6 - Prob. 23PCh. 6 - Sodium nitrite (NaNO2) reacted with 2-iodooctane...Ch. 6 - Prob. 25PCh. 6 - Prob. 26PCh. 6 - Prob. 27PCh. 6 - The reaction of 2,2-dimethyl-1-propanol with HBr...Ch. 6 - If the temperature is not kept below 25oC during...Ch. 6 - The reaction of cyclopentyl bromide with sodium...Ch. 6 - Prob. 31PCh. 6 - Prob. 32PCh. 6 - Write an equation, clearly showing the...Ch. 6 - Prob. 34PCh. 6 - Based on what we know about nucleophiles and...Ch. 6 - Prob. 36PCh. 6 - Prob. 37PCh. 6 - Prob. 38PCh. 6 - Prob. 39PCh. 6 - Prob. 40PCh. 6 - Prob. 41DSPCh. 6 - Prob. 42DSPCh. 6 - Prob. 43DSPCh. 6 - Prob. 44DSPCh. 6 - Prob. 45DSPCh. 6 - Prob. 46DSP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- what would a detailed step-by-step mechanism be for a reaction between bromine and trans-stilbene if the solvent, ethanol, is a stronger nucleophile than the bromide ions?arrow_forwardThe reaction that occurs when the benzaldehyde you have is reacted in a basic environment is called the Cannizzaro reaction, and when it is reacted with cyanide, it is called benzoin. Write down the reaction mechanisms of these reactions and explain why.arrow_forwardWrite a mechanism for the following reactionsarrow_forward
- A chemist needs an ether to use as a solvent for a reaction and wants to synthesize it in one step from two of the following available reagents: sodium ethoxide, bromomethane, potassium tert-butoxide, and 2-bromo-2-methylpropane. i) Which combination(s) will give a good yield of an ether? Illustrate, showing the mechanism of the reaction. ii) Illustrate with a mechanism the reaction of one of the combinations that will not yield an ether?arrow_forwardGive the major organic product(s) for each step of the following reactionarrow_forwardThe product formed as a result of the reaction between cyclohexanone and 3-butene-2-one write down the product and mechanism of the reaction.arrow_forward
- Write a mechanism that accounts for the formation of ethyl isopropyl ether as one of the products in the following reaction. CI OEt HCI EtOHarrow_forwardThe reaction that occurs when the benzaldehyde you have is reacted in a basic environment is called the Cannizzaro reaction, and when it is reacted with cyanide, it is called benzoin production. Write down the reaction mechanisms of these reactions and explain why.arrow_forwardPropanal and propanone react in a similar way with acidified aqueous potassium cyanide, KCN. For this reaction to occur reasonably quickly, the pH of the solution should be approximately 4. Draw a diagram to show the mechanism of the reaction of either propanal or propanone with acidified potassium cyanide.arrow_forward
- Possible alternative brominations include: Veratrole (1,2-dimethoxybenzene) to 1,2-dibromo-4,5-dimethoxybenzene; 4-Methylacetanilide to 2-bromo-4-methylacetanilide; 2-Methylacetanilide (made in experiment S.1) to 4-bromo-2-methylacetanilide; Vanillin to 5-bromovanillin; Acetanilide to 4-bromoacetanilide; a. b. C. d. e. EXPERIMENT S4: BROMINATION OF AROMATIC COMPOUNDS Certain other acetanilides made in experiment S.1 may also be used as precursors in this experiment. Estimated time: 1 afternoon Associated learning goals: Section 6, LG 6.6; Section 7, LG 7.2 and 7.4 Pre-lab report: complete the standard report form, and answer the following questions. In this experiment, molecular bromine (Br2) is generated from the redox reaction of potassium bromate with hydrobromic acid. Write a balanced equation for this process. Briefly outline the mechanism by which Br2 brominates your aromatic compound. Why do the bromine atoms end up at the positions indicated rather than anywhere else in the…arrow_forwardWhat mechanism does the reaction between benzyl chloride and triphenylphosphine go by? A lower yield of phosphonium salt is obtained in refluxing benzene than in xylene. Look up the boiling points for these solvents and explain why the difference in boiling points might influence of the yield? Why are the starting materials soluble in xylene but the product (phosphonium salt) is not soluble in xylene? We use petroleum ether to wash the phosphonium salt in filtration. Can we use water instead? Why?arrow_forwardwhich will proceed more easily at room temperature the bromination of cyclohexene or the bromination of benzene?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Coenzymes and cofactors; Author: CH15 SWAYAM Prabha IIT Madras;https://www.youtube.com/watch?v=bubY2Nm7hVM;License: Standard YouTube License, CC-BY
Aromaticity and Huckel's Rule; Author: Professor Dave Explains;https://www.youtube.com/watch?v=7-BguH4_WBQ;License: Standard Youtube License