
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Chapter 5.SE, Problem 56AP
Interpretation Introduction
a)
Interpretation:
Pro-R or Pro-S is to be identified in the given molecules.
Concept introduction:
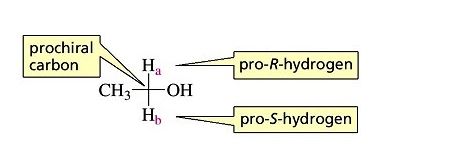
Interpretation Introduction
b)
Interpretation:
Pro-R or Pro-S is to be identified in the given molecules.
Concept introduction:

Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Part 4: Provide a detailed retrosynthetic analysis
and a plausible forward synthesis
the following molecule.
храдо
of
3A: Starting with benzocyclobutene, synthesize the naphthalene derivative below.
7. The addition of HBr to 2,5-dimethyl-2,4-heptadiene gives the same product, A, at both low and high temperatures.
Provide the structure of A and explain the kinetic and thermodynamic product are the same in this reaction.
HBr
-78°C
or
60°C
A
Chapter 5 Solutions
Organic Chemistry
Ch. 5.2 - Prob. 1PCh. 5.2 - Prob. 2PCh. 5.2 - Prob. 3PCh. 5.2 - Prob. 4PCh. 5.3 - Is cocaine (Worked Example 5-2) dextrorotatory or...Ch. 5.3 - Prob. 6PCh. 5.5 - Prob. 7PCh. 5.5 - Prob. 8PCh. 5.5 - Prob. 9PCh. 5.5 - Assign R or S configuration to the chirality...
Ch. 5.5 - Draw a tetrahedral representation of...Ch. 5.5 - Prob. 12PCh. 5.6 - One of the following molecules (a)–(d) is...Ch. 5.6 - Prob. 14PCh. 5.6 - Assign R or S configuration to each chirality...Ch. 5.7 - Prob. 16PCh. 5.7 - Which of the following have a meso form? (Recall...Ch. 5.7 - Does the following structure represent a meso...Ch. 5.8 - Prob. 19PCh. 5.8 - Prob. 20PCh. 5.9 - Prob. 21PCh. 5.11 - Prob. 22PCh. 5.11 - Prob. 23PCh. 5.11 - The lactic acid that builds up in tired muscles is...Ch. 5.11 - The aconitase-catalyzed addition of water to...Ch. 5.SE - Which of the following structures are identical?...Ch. 5.SE - Prob. 27VCCh. 5.SE - Prob. 28VCCh. 5.SE - Assign R or S configuration to each chirality...Ch. 5.SE - Prob. 30VCCh. 5.SE - Prob. 31APCh. 5.SE - Which of the following compounds are chiral? Draw...Ch. 5.SE - Prob. 33APCh. 5.SE - Eight alcohols have the formula C5H12O. Draw them....Ch. 5.SE - Draw compounds that fit the following...Ch. 5.SE - Prob. 36APCh. 5.SE - Prob. 37APCh. 5.SE - Prob. 38APCh. 5.SE - What is the stereochemical configuration of the...Ch. 5.SE - Prob. 40APCh. 5.SE - Prob. 41APCh. 5.SE - Prob. 42APCh. 5.SE - Prob. 43APCh. 5.SE - Assign R or S configuration to each chirality...Ch. 5.SE - Assign R or S configuration to each chirality...Ch. 5.SE - Prob. 46APCh. 5.SE - Assign R or S configuration to each chirality...Ch. 5.SE - Assign R or S configurations to the chirality...Ch. 5.SE - Assign R or S stereochemistry to the chirality...Ch. 5.SE - Prob. 50APCh. 5.SE - Draw examples of the following: (a) A meso...Ch. 5.SE - Prob. 52APCh. 5.SE - Prob. 53APCh. 5.SE - Prob. 54APCh. 5.SE - On reaction with hydrogen gas by a platinum...Ch. 5.SE - Prob. 56APCh. 5.SE - Prob. 57APCh. 5.SE - One of the steps in fat metabolism is the...Ch. 5.SE - The dehydration of citrate to yield cis-aconitate,...Ch. 5.SE - The first step in the metabolism of glycerol,...Ch. 5.SE - One of the steps in fatty-acid biosynthesis is the...Ch. 5.SE - Prob. 62APCh. 5.SE - Draw tetrahedral representations of the two...Ch. 5.SE - The naturally occurring form of the amino acid...Ch. 5.SE - Prob. 65APCh. 5.SE - Prob. 66APCh. 5.SE - Prob. 67APCh. 5.SE - Allenes are compounds with adjacent carbon-carbon...Ch. 5.SE - Prob. 69APCh. 5.SE - Prob. 70APCh. 5.SE - How many stereoisomers of...Ch. 5.SE - Draw both cis- and trans-1,4-dimethylcyclohexane...Ch. 5.SE - Draw both cis- and trans-1,3-dimethylcyclohexane...Ch. 5.SE - cis-1,2-Dimethylcyclohexane is optically inactive...Ch. 5.SE - Prob. 75APCh. 5.SE - Prob. 76APCh. 5.SE - Prob. 77AP
Knowledge Booster
Similar questions
- 3B: Convert the starting material into the chiral epoxytriol below. OH OH = OH OHarrow_forward3D: Convert the aromatic triketone to the 1,3,5-triethylcyclohexane shown below. ہوئےarrow_forwardIndicate how to find the energy difference between two levels in cm-1, knowing that its value is 2.5x10-25 joules.arrow_forward
- The gyromagnetic ratio (gamma) for 1H is 2.675x108 s-1 T-1. If the applied field is 1,409 T what will be the separation between nuclear energy levels?arrow_forwardChances Ad ~stract one 11. (10pts total) Consider the radical chlorination of 1,3-diethylcyclohexane depicted below. 4 • 6H total $4th total Statistical pro 21 total 2 H A 2H 래 • 4H totul < 3°C-H werkest bund - abstraction he leads to then mo fac a) (6pts) How many unique mono-chlorinated products can be formed and what are the structures for the thermodynamically and statistically favored products? рос 6 -વા J Number of Unique Mono-Chlorinated Products Thermodynamically Favored Product Statistically Favored Product b) (4pts) Draw the arrow pushing mechanism for the FIRST propagation step (p-1) for the formation of the thermodynamically favored product. Only draw the p-1 step. You do not need to include lone pairs of electrons. No enthalpy calculation necessary H H-Clarrow_forwardWhat is the lone pair or charge that surrounds the nitrogen here to give it that negative charge?arrow_forward
- Last Name, Firs Statifically more chances to abstract one of these 6H 11. (10pts total) Consider the radical chlorination of 1,3-diethylcyclohexane depicted below. 4 • 6H total $ 4th total 21 total 4H total ZH 2H Statistical H < 3°C-H werkst - product bund abstraction here leads to the mo favored a) (6pts) How many unique mono-chlorinated products can be formed and what are the structures for the thermodynamically and statistically favored products? Proclict 6 Number of Unique Mono-Chlorinated Products f Thermodynamically Favored Product Statistically Favored Product b) (4pts) Draw the arrow pushing mechanism for the FIRST propagation step (p-1) for the formation of the thermodynamically favored product. Only draw the p-1 step. You do not need to include lone pairs of electrons. No enthalpy calculation necessary 'H H-Cl Waterfoxarrow_forward2. (a) Many main group oxides form acidic solutions when added to water. For example solid tetraphosphorous decaoxide reacts with water to produce phosphoric acid. Write a balanced chemical equation for this reaction. (b) Calcium phosphate reacts with silicon dioxide and carbon graphite at elevated temperatures to produce white phosphorous (P4) as a gas along with calcium silicate (Silcate ion is SiO3²-) and carbon monoxide. Write a balanced chemical equation for this reaction.arrow_forwardI find the solution way too brief and unsatisfactory as it does not clearly explain the solution provided in the problem.arrow_forward
- Please correct answer and don't used hand raitingarrow_forwardPlease correct answer and don't used hand raitingarrow_forwardthis is an organic chemistry question please answer accordindly!! please post the solution in your hand writing not an AI generated answer please draw the figures and structures if needed to support your explanation hand drawn only!!!! answer the question in a very simple and straight forward manner thanks!!!!! im reposting this please solve all parts and draw it not just word explanations!!arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning