
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 5.7, Problem 18P
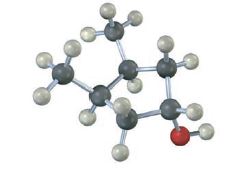
Does the following structure represent a meso compound? If so, indicate the symmetry plane.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Consider the structure of 1-bromo-2-fluoroethane.
Part 1 of 2
Draw the Newman projection for the anti conformation of 1-bromo-2-fluoroethane, viewed down the C1-C2 bond.
✡
ぬ
Part 2 of 2
H
H
F
Br
H
H
☑
Draw the Newman projection for the gauche conformation of 1-bromo-2-fluoroethane, viewed down the C1-C2 bond.
H
F
Br
H
H
Please help me answer this question. I don't understand how or where the different reagents will attach and it's mostly due to the wedge bond because I haven't seen a problem like this before. Please provide a detailed explanation and a drawing showing how it can happen and what the final product will look like.
Which of the following compounds is the most acidic in the gas phase?
Group of answer choices
H2O
SiH4
HBr
H2S
Chapter 5 Solutions
Organic Chemistry
Ch. 5.2 - Prob. 1PCh. 5.2 - Prob. 2PCh. 5.2 - Prob. 3PCh. 5.2 - Prob. 4PCh. 5.3 - Is cocaine (Worked Example 5-2) dextrorotatory or...Ch. 5.3 - Prob. 6PCh. 5.5 - Prob. 7PCh. 5.5 - Prob. 8PCh. 5.5 - Prob. 9PCh. 5.5 - Assign R or S configuration to the chirality...
Ch. 5.5 - Draw a tetrahedral representation of...Ch. 5.5 - Prob. 12PCh. 5.6 - One of the following molecules (a)–(d) is...Ch. 5.6 - Prob. 14PCh. 5.6 - Assign R or S configuration to each chirality...Ch. 5.7 - Prob. 16PCh. 5.7 - Which of the following have a meso form? (Recall...Ch. 5.7 - Does the following structure represent a meso...Ch. 5.8 - Prob. 19PCh. 5.8 - Prob. 20PCh. 5.9 - Prob. 21PCh. 5.11 - Prob. 22PCh. 5.11 - Prob. 23PCh. 5.11 - The lactic acid that builds up in tired muscles is...Ch. 5.11 - The aconitase-catalyzed addition of water to...Ch. 5.SE - Which of the following structures are identical?...Ch. 5.SE - Prob. 27VCCh. 5.SE - Prob. 28VCCh. 5.SE - Assign R or S configuration to each chirality...Ch. 5.SE - Prob. 30VCCh. 5.SE - Prob. 31APCh. 5.SE - Which of the following compounds are chiral? Draw...Ch. 5.SE - Prob. 33APCh. 5.SE - Eight alcohols have the formula C5H12O. Draw them....Ch. 5.SE - Draw compounds that fit the following...Ch. 5.SE - Prob. 36APCh. 5.SE - Prob. 37APCh. 5.SE - Prob. 38APCh. 5.SE - What is the stereochemical configuration of the...Ch. 5.SE - Prob. 40APCh. 5.SE - Prob. 41APCh. 5.SE - Prob. 42APCh. 5.SE - Prob. 43APCh. 5.SE - Assign R or S configuration to each chirality...Ch. 5.SE - Assign R or S configuration to each chirality...Ch. 5.SE - Prob. 46APCh. 5.SE - Assign R or S configuration to each chirality...Ch. 5.SE - Assign R or S configurations to the chirality...Ch. 5.SE - Assign R or S stereochemistry to the chirality...Ch. 5.SE - Prob. 50APCh. 5.SE - Draw examples of the following: (a) A meso...Ch. 5.SE - Prob. 52APCh. 5.SE - Prob. 53APCh. 5.SE - Prob. 54APCh. 5.SE - On reaction with hydrogen gas by a platinum...Ch. 5.SE - Prob. 56APCh. 5.SE - Prob. 57APCh. 5.SE - One of the steps in fat metabolism is the...Ch. 5.SE - The dehydration of citrate to yield cis-aconitate,...Ch. 5.SE - The first step in the metabolism of glycerol,...Ch. 5.SE - One of the steps in fatty-acid biosynthesis is the...Ch. 5.SE - Prob. 62APCh. 5.SE - Draw tetrahedral representations of the two...Ch. 5.SE - The naturally occurring form of the amino acid...Ch. 5.SE - Prob. 65APCh. 5.SE - Prob. 66APCh. 5.SE - Prob. 67APCh. 5.SE - Allenes are compounds with adjacent carbon-carbon...Ch. 5.SE - Prob. 69APCh. 5.SE - Prob. 70APCh. 5.SE - How many stereoisomers of...Ch. 5.SE - Draw both cis- and trans-1,4-dimethylcyclohexane...Ch. 5.SE - Draw both cis- and trans-1,3-dimethylcyclohexane...Ch. 5.SE - cis-1,2-Dimethylcyclohexane is optically inactive...Ch. 5.SE - Prob. 75APCh. 5.SE - Prob. 76APCh. 5.SE - Prob. 77AP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which of the following is the most acidic transition metal cation? Group of answer choices Fe3+ Sc3+ Mn4+ Zn2+arrow_forwardBased on the thermodynamics of acetic acid dissociation discussed in Lecture 2-5, what can you conclude about the standard enthalpy change (ΔHo) of acid dissociation for HCl? Group of answer choices You cannot arrive at any of the other three conclusions It is a positive value It is more negative than −0.4 kJ/mol It equals −0.4 kJ/molarrow_forwardPLEASE HELP URGENT!arrow_forward
- Draw the skeletal structure corresponding to the following IUPAC name: 7-isopropyl-3-methyldecanearrow_forwardWhich of the following oxyacids is the weakest? Group of answer choices H2SeO3 Si(OH)4 H2SO4 H3PO4arrow_forwardAdd conditions above and below the arrow that turn the reactant below into the product below in a single transformation. + More... If you need to write reagents above and below the arrow that have complex hydrocarbon groups in them, there is a set of standard abbreviations you can use. More... T H,N NC Datarrow_forward
- Indicate the order of basicity of primary, secondary and tertiary amines.arrow_forward> Classify each of the following molecules as aromatic, antiaromatic, or nonaromatic. Cl Z- N O aromatic O antiaromatic O nonaromatic O aromatic O antiaromatic O nonaromatic O aromatic ○ antiaromatic nonaromaticarrow_forwardPlease help me answer this question. I don't understand how or even if this can happen in a single transformation. Please provide a detailed explanation and a drawing showing how it can happen in a single transformation. Add the necessary reagents and reaction conditions above and below the arrow in this organic reaction. If the products can't be made from the reactant with a single transformation, check the box under the drawing area instead.arrow_forward
- 2) Draw the correct chemical structure (using line-angle drawings / "line structures") from their given IUPAC name: a. (E)-1-chloro-3,4,5-trimethylhex-2-ene b. (Z)-4,5,7-trimethyloct-4-en-2-ol C. (2E,6Z)-4-methylocta-2,6-dienearrow_forwardපිපිම Draw curved arrows to represent the flow of electrons in the reaction on the left Label the reactants on the left as either "Acid" or "Base" (iii) Decide which direction the equilibrium arrows will point in each reaction, based on the given pk, values (a) + H-O H 3-H + (c) H" H + H****H 000 44-00 NH₂ (e) i Дон OH Ө NHarrow_forward3) Label the configuration in each of the following alkenes as E, Z, or N/A (for non-stereogenic centers). 00 E 000 N/A E Br N/A N/A (g) E N/A OH E (b) Oz N/A Br (d) 00 E Z N/A E (f) Oz N/A E (h) Z N/Aarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Group Theory - Learn like Expert with 3D animation | Introduction for Beginners | ONE Chemistry; Author: One Chemistry;https://www.youtube.com/watch?v=Lz2ih8fkgDs;License: Standard YouTube License, CC-BY