
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 5.6, Problem 13P
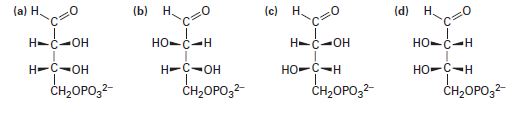
One of the following molecules (a)–(d) is d-erythrose 4-phosphate, an intermediate in the Calvin photosynthetic cycle by which plants incorporate CO2 into carbohydrates. If d-erythrose 4-phosphate has R stereochemistry at both chirality centers, which of the structures is it? Which of the remaining three structures is the enantiomer of d-erythrose 4-phosphate, and which are diastereomers?
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Please answer the questions and provide detailed explanation. Please also include the Hydrogens that are on the molecule to show how many signals there are.
Capp aktiv.com
Part of Speech Table for Assi
x
Aktiv Learning App
K
Curved arrows are used to illustrate the flow of electrons. Using
the provided starting and product structures, draw the curved
electron-pushing arrows for the following reaction or
mechanistic step(s).
Be sure to account for all bond-breaking and bond-making
steps.
Problem 232 of 10
10:
Mg
Select to Add Arrows
Br
O
H
:0
CI:O
H
Mg
THE
+
dy
Undo
Reset Done
Br
Please answer the question and provide a detailed drawing of the structure. If there will not be a new C – C bond, then the box under the drawing area will be checked.
Will the following reaction make a molecule with a new C – C bond as its major product:
Draw the major organic product or products, if the reaction will work. Be sure you use wedge and dash bonds if necessary, for example to distinguish between major products with different stereochemistry.
Chapter 5 Solutions
Organic Chemistry
Ch. 5.2 - Prob. 1PCh. 5.2 - Prob. 2PCh. 5.2 - Prob. 3PCh. 5.2 - Prob. 4PCh. 5.3 - Is cocaine (Worked Example 5-2) dextrorotatory or...Ch. 5.3 - Prob. 6PCh. 5.5 - Prob. 7PCh. 5.5 - Prob. 8PCh. 5.5 - Prob. 9PCh. 5.5 - Assign R or S configuration to the chirality...
Ch. 5.5 - Draw a tetrahedral representation of...Ch. 5.5 - Prob. 12PCh. 5.6 - One of the following molecules (a)–(d) is...Ch. 5.6 - Prob. 14PCh. 5.6 - Assign R or S configuration to each chirality...Ch. 5.7 - Prob. 16PCh. 5.7 - Which of the following have a meso form? (Recall...Ch. 5.7 - Does the following structure represent a meso...Ch. 5.8 - Prob. 19PCh. 5.8 - Prob. 20PCh. 5.9 - Prob. 21PCh. 5.11 - Prob. 22PCh. 5.11 - Prob. 23PCh. 5.11 - The lactic acid that builds up in tired muscles is...Ch. 5.11 - The aconitase-catalyzed addition of water to...Ch. 5.SE - Which of the following structures are identical?...Ch. 5.SE - Prob. 27VCCh. 5.SE - Prob. 28VCCh. 5.SE - Assign R or S configuration to each chirality...Ch. 5.SE - Prob. 30VCCh. 5.SE - Prob. 31APCh. 5.SE - Which of the following compounds are chiral? Draw...Ch. 5.SE - Prob. 33APCh. 5.SE - Eight alcohols have the formula C5H12O. Draw them....Ch. 5.SE - Draw compounds that fit the following...Ch. 5.SE - Prob. 36APCh. 5.SE - Prob. 37APCh. 5.SE - Prob. 38APCh. 5.SE - What is the stereochemical configuration of the...Ch. 5.SE - Prob. 40APCh. 5.SE - Prob. 41APCh. 5.SE - Prob. 42APCh. 5.SE - Prob. 43APCh. 5.SE - Assign R or S configuration to each chirality...Ch. 5.SE - Assign R or S configuration to each chirality...Ch. 5.SE - Prob. 46APCh. 5.SE - Assign R or S configuration to each chirality...Ch. 5.SE - Assign R or S configurations to the chirality...Ch. 5.SE - Assign R or S stereochemistry to the chirality...Ch. 5.SE - Prob. 50APCh. 5.SE - Draw examples of the following: (a) A meso...Ch. 5.SE - Prob. 52APCh. 5.SE - Prob. 53APCh. 5.SE - Prob. 54APCh. 5.SE - On reaction with hydrogen gas by a platinum...Ch. 5.SE - Prob. 56APCh. 5.SE - Prob. 57APCh. 5.SE - One of the steps in fat metabolism is the...Ch. 5.SE - The dehydration of citrate to yield cis-aconitate,...Ch. 5.SE - The first step in the metabolism of glycerol,...Ch. 5.SE - One of the steps in fatty-acid biosynthesis is the...Ch. 5.SE - Prob. 62APCh. 5.SE - Draw tetrahedral representations of the two...Ch. 5.SE - The naturally occurring form of the amino acid...Ch. 5.SE - Prob. 65APCh. 5.SE - Prob. 66APCh. 5.SE - Prob. 67APCh. 5.SE - Allenes are compounds with adjacent carbon-carbon...Ch. 5.SE - Prob. 69APCh. 5.SE - Prob. 70APCh. 5.SE - How many stereoisomers of...Ch. 5.SE - Draw both cis- and trans-1,4-dimethylcyclohexane...Ch. 5.SE - Draw both cis- and trans-1,3-dimethylcyclohexane...Ch. 5.SE - cis-1,2-Dimethylcyclohexane is optically inactive...Ch. 5.SE - Prob. 75APCh. 5.SE - Prob. 76APCh. 5.SE - Prob. 77AP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please answer the question and provide a detailed drawing of the structure. If there will not be a new C – C bond, then the box under the drawing area will be checked. Will the following reaction make a molecule with a new C – C bond as its major product: Draw the major organic product or products, if the reaction will work. Be sure you use wedge and dash bonds if necessary, for example to distinguish between major products with different stereochemistry.arrow_forwardPlease do not use AI. AI cannot "see" the molecules properly, and it therefore gives the wrong answer while giving incorrect descriptions of the visual images we're looking at. All of these compounds would be produced (I think). In my book, I don't see any rules about yield in this case, like explaining that one product would be present in less yield for this reason or that reason. Please explain why some of these produce less yield than others.arrow_forwardPlease answer the question and provide detailed explanations.arrow_forward
- All of these compounds would be produced (I think). In my book, I don't see any rules about yield in this case, like explaining that one product would be present in less yield for this reason or that reason. Please explain why some of these produce less yield than others.arrow_forward5. Fill in the missing molecules in the following reaction pathway. TMSO Heat + CI then HF O₂N (1.0 equiv) AICI 3 OMearrow_forwarde. O₂N NO2 1. excess H2, Pd/C 2. excess NaNO2, HCI 3. excess CuCNarrow_forward
- Help with a periodic table task.' Procedure Part 1: Customizing a Periodic Table Use a textbook or other valid source to determine which elements are metals, nonmetals, metalloids (called semimetals in some texts), alkali metals, alkaline earth metals, transition metals, halogens, and noble gases. Download and print a copy of the Periodic Table of Elements. Use colored pencils, colorful highlighters, or computer drawing tools to devise a schematic for designating each of the following on the periodic table: Group numbers Period number Labels for these groups: alkali metals, alkaline earth metals, transition metals, inner transition metals (lanthanides and actinides), other metals, metalloids (semimetals), other nonmetals, halogens, and noble gases Metals, nonmetals, and metalloids Note: Write the group and period numbers and color/highlight each element for categorization. Be sure to include a key for the schematic. Take a photo of the completed periodic table and upload the…arrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forwardCan you explain these two problems for mearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole
Enzymes - Effect of cofactors on enzyme; Author: Tutorials Point (India) Ltd;https://www.youtube.com/watch?v=AkAbIwxyUs4;License: Standard YouTube License, CC-BY
Enzyme Catalysis Part-I; Author: NPTEL-NOC IITM;https://www.youtube.com/watch?v=aZE740JWZuQ;License: Standard Youtube License