
A tank with an internal volume of 1 m3 contains air at 800 kPa and 25°C. A valve on the tank is opened, allowing air to escape, and the pressure inside quickly drops to 150 kPa, at which point the valve is closed. Assume there is negligible heat transfer from the tank to the air left in the tank.
- (a) Using the approximation he ≈ constant = he,avg = 0.5 (h1 + h2), calculate the mass withdrawn during the process.
- (b) Consider the same process but broken into two parts. That is, consider an intermediate state at P2 = 400 kPa, calculate the mass removed during the process from P1 = 800 kPa to P2 and then the mass removed during the process from P2 to P3 = 150 kPa, using the type of approximation used in part (a), and add the two to get the total mass removed.
- (c) Calculate the mass removed if the variation of he is accounted for.
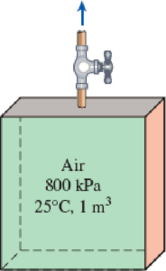
FIGURE P5–185
(a)
The mass withdrawn during the process.
Answer to Problem 185RP
The mass withdrawn during the process is
Explanation of Solution
Write the equation of mass balance.
Here, the inlet mass is
The change in mass of the system for the control volume is expressed as,
Here, the suffixes 1 and 2 indicates the initial and final states of the system.
Consider the tank as the control volume. Initially the tank is filled with air and the valve is in closed position, further no other mass is allowed to enter the tank. Hence, the inlet mass is neglected i.e.
Rewrite the Equation (I) as follows.
Write the formula for initial and final mass of air present in the tank.
Here, the mass of air is
Write the energy balance equation.
Here, the heat transfer is
When the valve is opened and air starts escape from the tank. Neglect the heat transfer and work done i.e.
The Equation (V) reduced as follows.
The enthalpy and internal energy in terms of temperature and specific heats are expressed as follows.
Rewrite the Equation (VI) as follows.
The temperature of the air while exiting the tank is considered as the average temperature of initial and final temperatures.
Refer Table A-1, “Molar mass, gas constant, and critical-point properties”.
The gas constant
Refer Table A-2b, “Ideal-gas specific heats of various common gases”.
The specific heat at constant pressure
Conclusion:
Substitute
Substitute
Substitute
Use Engineering Equation Solver (EES) or online calculator to solve the Equation (VIII) and obtain the value of
Substitute
Substitute
Thus, the mass withdrawn during the process is
(b)
The mass withdrawn during the pressure reduced from
Answer to Problem 185RP
The total mass withdrawn during the process 1-3 is
Explanation of Solution
Consider Process 1-2:
The pressure drop from
Substitute
Substitute
Substitute
Use Engineering Equation Solver (EES) or online calculator to solve the Equation (IX) and obtain the value of
Substitute
Substitute
Thus, the mass withdrawn during the process 1-2 is
Consider Process 2-3:
The pressure drop from
Here,
Substitute
Substitute
Substitute
Use Engineering Equation Solver (EES) or online calculator to solve the Equation (X) and obtain the value of
Substitute
Substitute
Thus, the mass withdrawn during the process 2-3 is
The total mass withdrawn during the process 1-3 is as follows.
Thus, the total mass withdrawn during the process 1-3 is
(c)
The mass withdrawn during the process if there is variation in
Answer to Problem 185RP
The mass withdrawn during the process is
Explanation of Solution
Write the general mass balance equation.
Here, the inlet mass flow rate is
Refer Equation (XI).
Write the mass balance equation for the given system.
Rewrite the Equation (XII) as follows.
Write the general energy rate balance equation.
Here, the rate of total energy in is
The system is at steady state. Hence, the rate of change in net energy of the system becomes zero.
Refer Equation (XIII).
Write the energy balance equation for the given system.
Here, the mass is
Substitute
The enthalpy and internal energy is expressed as follows.
Substitute
The mass of air in terms ideal gas is expressed as follows.
Rewrite the Equation (XVI) as follows.
Using
Substitute
Here,
Integrate the Equation (XVIII) at the initial-1 and final-2 states.
Refer Table A-2(a), “Ideal-gas specific heats of various common gases”.
The specific heat ratio
Conclusion:
Substitute
Substitute
Substitute
Thus, the mass withdrawn during the process is
Want to see more full solutions like this?
Chapter 5 Solutions
EBK THERMODYNAMICS: AN ENGINEERING APPR
Additional Engineering Textbook Solutions
Fluid Mechanics: Fundamentals and Applications
Starting Out with C++ from Control Structures to Objects (9th Edition)
Management Information Systems: Managing The Digital Firm (16th Edition)
Automotive Technology: Principles, Diagnosis, And Service (6th Edition) (halderman Automotive Series)
Java: An Introduction to Problem Solving and Programming (8th Edition)
Starting Out with Programming Logic and Design (5th Edition) (What's New in Computer Science)
- First monthly exam Gas dynamics Third stage Q1/Water at 15° C flow through a 300 mm diameter riveted steel pipe, E-3 mm with a head loss of 6 m in 300 m length. Determine the flow rate in pipe. Use moody chart. Q2/ Assume a car's exhaust system can be approximated as 14 ft long and 0.125 ft-diameter cast-iron pipe ( = 0.00085 ft) with the equivalent of (6) regular 90° flanged elbows (KL = 0.3) and a muffler. The muffler acts as a resistor with a loss coefficient of KL= 8.5. Determine the pressure at the beginning of the exhaust system (pl) if the flowrate is 0.10 cfs, and the exhaust has the same properties as air.(p = 1.74 × 10-3 slug/ft³, u= 4.7 x 10-7 lb.s/ft²) Use moody chart (1) MIDAS Kel=0.3 Q3/Liquid ammonia at -20°C is flowing through a 30 m long section of a 5 mm diameter copper tube(e = 1.5 × 10-6 m) at a rate of 0.15 kg/s. Determine the pressure drop and the head losses. .μ= 2.36 × 10-4 kg/m.s)p = 665.1 kg/m³arrow_forward2/Y Y+1 2Cp Q1/ Show that Cda Az x P1 mactual Cdf Af R/T₁ 2pf(P1-P2-zxgxpf) Q2/ A simple jet carburetor has to supply 5 Kg of air per minute. The air is at a pressure of 1.013 bar and a temperature of 27 °C. Calculate the throat diameter of the choke for air flow velocity of 90 m/sec. Take velocity coefficient to be 0.8. Assume isentropic flow and the flow to be compressible. Quiz/ Determine the air-fuel ratio supplied at 5000 m altitude by a carburetor which is adjusted to give an air-fuel ratio of 14:1 at sea level where air temperature is 27 °C and pressure is 1.013 bar. The temperature of air decreases with altitude as given by the expression The air pressure decreases with altitude as per relation h = 19200 log10 (1.013), where P is in bar. State any assumptions made. t = ts P 0.0065harrow_forward36 2) Use the method of MEMBERS to determine the true magnitude and direction of the forces in members1 and 2 of the frame shown below in Fig 3.2. 300lbs/ft member-1 member-2 30° Fig 3.2. https://brightspace.cuny.edu/d21/le/content/433117/viewContent/29873977/Viewarrow_forward
- Can you solve this for me?arrow_forward5670 mm The apartment in the ground floor of three floors building in Fig. in Baghdad city. The details of walls, roof, windows and door are shown. The window is a double glazing and air space thickness is 1.3cm Poorly Fitted-with Storm Sash with wood strip and storm window of 0.6 cm glass thickness. The thickness of door is 2.5 cm. The door is Poor Installation. There are two peoples in each room. The height of room is 280 cm. assume the indoor design conditions are 25°C DBT and 50 RH, and moisture content of 8 gw/kga. The moisture content of outdoor is 10.5 gw/kga. Calculate heat gain for living room : الشقة في الطابق الأرضي من مبنى ثلاثة طوابق في مدينة بغداد يظهر في مخطط الشقة تفاصيل الجدران والسقف والنوافذ والباب. النافذة عبارة عن زجاج مزدوج وسمك الفراغ الهوائي 1.3 سم ضعيف الاحكام مع ساتر حماية مع إطار خشبي والنافذة بسماكة زجاج 0.6 سم سماكة الباب 2.5 سم. الباب هو تركيب ضعيف هناك شخصان في كل غرفة. ارتفاع الغرفة 280 سم. افترض أن ظروف التصميم الداخلي هي DBT25 و R50 ، ومحتوى الرطوبة 8…arrow_forwardHow do i solve this problem?arrow_forward
- Q4/ A compressor is driven motor by mean of a flat belt of thickness 10 mm and a width of 250 mm. The motor pulley is 300 mm diameter and run at 900 rpm and the compressor pulley is 1500 mm diameter. The shaft center distance is 1.5 m. The angle of contact of the smaller pulley is 220° and on the larger pulley is 270°. The coefficient of friction between the belt and the small pulley is 0.3, and between the belt and the large pulley is 0.25. The maximum allowable belt stress is 2 MPa and the belt density is 970 kg/m³. (a) What is the power capacity of the drive and (b) If the small pulley replaced by V-grooved pulley of diameter 300 mm, grooved angle of 34° and the coefficient of friction between belt and grooved pulley is 0.35. What will be the power capacity in this case, assuming that the diameter of the large pulley remain the same of 1500 mm.arrow_forwardYou are tasked with designing a power drive system to transmit power between a motor and a conveyor belt in a manufacturing facility as illustrated in figure. The design must ensure efficient power transmission, reliability, and safety. Given the following specifications and constraints, design drive system for this application: Specifications: Motor Power: The electric motor provides 10 kW of power at 1,500 RPM. Output Speed: The output shaft should rotate at 150 rpm. Design Decisions: Transmission ratio: Determine the necessary drive ratio for the system. Shaft Diameter: Design the shafts for both the motor and the conveyor end. Material Selection: Choose appropriate materials for the gears, shafts. Bearings: Select suitable rolling element bearings. Constraints: Space Limitation: The available space for the gear drive system is limited to a 1-meter-long section. Attribute 4 of CEP Depth of knowledge required Fundamentals-based, first principles analytical approach…arrow_forward- | العنوان In non-continuous dieless drawing process for copper tube as shown in Fig. (1), take the following data: Do-20mm, to=3mm, D=12mm, ti/to=0.6 and v.-15mm/s. Calculate: (1) area reduction RA, (2) drawing velocity v. Knowing that: ti: final thickness V. Fig. (1) ofthrearrow_forward
- A direct extrusion operation produces the cross section shown in Fig. (2) from an aluminum billet whose diameter 160 mm and length - 700 mm. Determine the length of the extruded section at the end of the operation if the die angle -14° 60 X Fig. (2) Note: all dimensions in mm.arrow_forwardFor hot rolling processes, show that the average strain rate can be given as: = (1+5)√RdIn(+1)arrow_forward: +0 usão العنوان on to A vertical true centrifugal casting process is used to produce bushings that are 250 mm long and 200 mm in outside diameter. If the rotational speed during solidification is 500 rev/min, determine the inside radii at the top and bottom of the bushing if R-2R. Take: -9.81 mis ۲/۱ ostrararrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





