
Organic Chemistry, Books a la Carte Edition (9th Edition)
9th Edition
ISBN: 9780134160382
Author: Leroy G. Wade, Jan W. Simek
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 5.10A, Problem 5.17P
Draw a Fischer projection for each compound. Remember that the cross represents an asymmetric carbon atom, and the carbon chain should be along the vertical, with the IUPAC numbering from top to bottom.
- a. (S)-propane-1,2-
diol - b. (R)-2-bromobutan 1-ol
- c. (S)-1,2-dibromobutane
- d. (R)-butan-2-ol
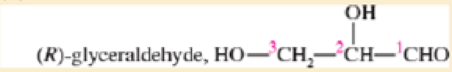
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
5. Draw the structure of (S)-1-bromo-1-chloropropane. Take care to indicate the three-
dimensional stereochemistry properly.
6. How many chiral centers does the following compound contain?
CH3
7. Label each chiral carbon in the molecule below as having R or S configuration.
HO₂C H
F.
H3C
H
CH₂CH3
1. Draw the two conformations of trans-1-bromo-3-methylcyclohexane using both the Newman
projection and the chair drawing. Calculate the energies of each. What ratio of the two
conformers will be present at equilibrium?
2. Draw a structure of a chiral alcohol.
Glucose is a simple sugar with five substituents bonded to a sixmembered ring.a.Using a chair representation, draw the most stable arrangement of these substituents on the six-membered ring.
b.Convert this representation to one that uses a hexagon with wedges and dashed wedges.
c.Draw a constitutional isomer of glucose.
d.Draw a stereoisomer that has an axial OH group on one carbon.
Chapter 5 Solutions
Organic Chemistry, Books a la Carte Edition (9th Edition)
Ch. 5.2 - Determine whether the following objects are chiral...Ch. 5.2A - Prob. 5.2PCh. 5.2B - Prob. 5.3PCh. 5.2B - Prob. 5.4PCh. 5.2C - Prob. 5.5PCh. 5.3 - Prob. 5.6PCh. 5.3 - Prob. 5.7PCh. 5.4D - Prob. 5.8PCh. 5.4D - Prob. 5.9PCh. 5.4D - Prob. 5.10P
Ch. 5.5 - Prob. 5.11PCh. 5.7 - When optically pure (R)-2-bromobutane is heated...Ch. 5.7 - Prob. 5.13PCh. 5.8 - Prob. 5.14PCh. 5.9B - Draw three-dimensional representations of the...Ch. 5.10A - For each sot of examples, make a model of the...Ch. 5.10A - Draw a Fischer projection for each compound....Ch. 5.10B - Prob. 5.18PCh. 5.10C - For each Fischer projection, label each asymmetric...Ch. 5.11C - Prob. 5.20PCh. 5.13 - Prob. 5.21PCh. 5.13 - Prob. 5.22PCh. 5.15 - Prob. 5.23PCh. 5.16A - Prob. 5.24PCh. 5 - The following four structures are naturally...Ch. 5 - For each structure, 1. star () any asymmetric...Ch. 5 - Prob. 5.27SPCh. 5 - Prob. 5.28SPCh. 5 - Prob. 5.29SPCh. 5 - Prob. 5.30SPCh. 5 - Prob. 5.31SPCh. 5 - Prob. 5.32SPCh. 5 - Prob. 5.33SPCh. 5 - Prob. 5.34SPCh. 5 - For each structure, 1. draw all the stereoisomers....Ch. 5 - Prob. 5.36SPCh. 5 - Prob. 5.37SPCh. 5 - 3,4-Dimethylpent-1-ene has the formula...Ch. 5 - A graduate student was studying enzymatic...Ch. 5 - Prob. 5.40SPCh. 5 - Prob. 5.41SP
Additional Science Textbook Solutions
Find more solutions based on key concepts
Calculate the lattice energy of CaCl2 using a Born-Haber cycle and data from Appendices F and L and Table 7.5. ...
Chemistry & Chemical Reactivity
Practice Exercise 1
Which of the following factors determines the size of an atom? a. the volume of the nucleus...
Chemistry: The Central Science (14th Edition)
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, & Biological Chemistry
Real walls are never totally adiabatic. Use your experience to order the following walls in increasing order wi...
Thermodynamics, Statistical Thermodynamics, & Kinetics
Practice Exercise 1
Which of the following factors determines the size of an atom? a. the volume of the nucleus...
Chemistry: The Central Science (13th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Classify each compound as identical to A or its enantiomer. CHO CHO H OH CHO a. CH,CH-C--OH CumCH,CH3 H OH b. d. HO C. ČH,CH3 н он CH;CH CHO CHO Aarrow_forward6. Indicate whether the circled carbon in each of these is chiral a. CH3-CH-CH2-CH3 b. CH3-CH2-C-CH3 Cl c. CH3-CH2-CH-OH d. Br CH2 ©H H,C CH2 CH3 HC. CH2 Br CHarrow_forwardUsing the cyclohexane with the C’s numbered as shown, draw a chair form that fits each description. a. The ring has an axial CH3 group at C1 and an equatorial OH on C2. b. The ring has an equatorial CH3 group on C6 and an axial OH group on C4. c. The ring has equatorial OH groups on C1, C2, and C5.arrow_forward
- Draw a chair conformation of cyclohexane with one CH3CH2 group and one CH3 group that fits each description. a.a 1,1-disubstituted cyclohexane with an axial CH3CH2 group b. a cis-1,2-disubstituted cyclohexane with an axial CH3 group c. a trans-1,3-disubstituted cyclohexane with an equatorial CH3 group d. a trans-1,4-disubstituted cyclohexane with an equatorial CH3CH2 group.arrow_forwardDraw a chair conformation of cyclohexane with one CH3CH2 group and one CH3 group that fits each description. a. a 1,1-disubstituted cyclohexane with an axial CH3CH2 group b. a cis-1,2-disubstituted cyclohexane with an axial CH3 group c. a trans-1,3-disubstituted cyclohexane with an equatorial CH3 group d. a trans-1,4-disubstituted cyclohexane with an equatorial CH3CH2 grouparrow_forwardConsider CH3-CH(OH)-CH(OH)(Br). a.How many stereogeniccenters are in the molecule? b.How many stereoisomers are there for the compound? c.Draw the Fischer projection for each of the stereoisomer. Label each using I, II, etc. d.Which pairs are enantiomers? Which are diastereomers? e.Determine the absolute configuration of each chiral center in one pair of diastereomer.arrow_forward
- Consider 1,2-dimethylcyclohexane. a.Draw structures for the cis and trans isomers using a hexagon for the sixmembered ring. b. Draw the two possible chair conformations for the cis isomer. Which conformation, if either, is more stable? c. Draw the two possible chair conformations for the trans isomer. Which conformation, if either, is more stable? d.Which isomer, cis or trans, is more stable and why?arrow_forward4. Do the following structures represent the same molecules or pairs of enantiomers? Additionally, convert the perspective structures to Fischer projections and vice versa. a. b. C. d. 11 CH HOH₂CH₂C-COH CH₂Br H₂C-CCI CH₂CH3 CH₂Br H-OH CH3 CI HỌC CHỊCH H CH₂CH3 CI HẠCH,C-C 'CH, CH₂Br H₂CH₂C-CH₂CH₂OH OH H HO-CH3 CH₂Br CH3 0-5-6-5 H+CI CH CH₂CH3arrow_forward10. Draw the enantiomer of the following molecule. Circle each chiral carbon in the original molecule. a. HO Но H- H- FHO- H- HO- HO,arrow_forward
- Draw the mirror image of each compound. Label each molecule as chiral or achiral.arrow_forwardUsing the cyclohexane with the C's numbered as shown, draw a chair form that fits each description.a.The ring has an axial CH3 group at C1 and an equatorial OH on C2. b.The ring has an equatorial CH3 group on C6 and an axial OH group on C4. c.The ring has equatorial OH groups on C1, C2, and C5.arrow_forward1a. How many stereogenic centers are present 1c. Draw a three-dimensional structure of a in the structure below? Indicate them with asterisk(s). How many stereoisomers stereoisomers are possible? chiral compound with the molecular formula of C4H4Cl₂ that does not have a stereogenic carbon. In addition, draw the enantiomer of this compound. Number of stereogenic centers: Number of stereoisomers possible: 1b. Draw one of the two most stable stereoisomers of the compound in 1a using a planar structure with wedges and dashes. Now draw it in its preferred chair conformation. 1d. Draw two meso compounds with the molecular formula of C7H14.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY