
Interpretation:
It is to be explained that the
Concept introduction:
The reaction of methyl and primary alcohols with hydrogen halide proceeds through
The
The reaction of tertiary alcohol with hydrogen halide proceeds through
The
The slow step in the
The carbocation (positively charged carbon) is
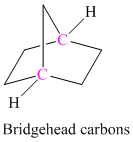
The carbocation at bridgehead carbon is unstable.
The carbon atom which is a part of two or more rings in polycyclic compounds is as shown below:
Want to see the full answer?
Check out a sample textbook solution
Chapter 5 Solutions
Organic Chemistry - Standalone book
- How many isomers (structural, geometric, and stereo) have the formula C5H10, and have "cyclo" in their name? (i.e. they contain a ring) 4 06 07 5arrow_forwarda-pinene When a-pinene reacts with 1. BH3; 2. H₂O2, NaOH, H₂O 4 possible cis,trans isomers can be formed, but one of these is formed more readily than the others. Draw the structure of this preferred isomer. • Use the wedge/hash bond tools to indicate stereochemistry where it exists. • To aid you in your drawing, the structure of a-pinene is provided for you in the drawing palette.arrow_forwardWhich will be more stable, cis or trans-1,4-tert-butylcyclohexane? Explain by drawing their structures?arrow_forward
- Draw the structure of the following compounds. Use line-angle structural formula. 1-bromo-4-ethyl-5- methylcyclohexane 3,7-dimethyl-5-propylnonae 5-chloro-1- ethylspiro[2.5]octane 2-bromo-4- methylbicyclo[3.2.1]octane * 5-cyclobutyl-2-cyclopropyl- 6,8-diethyl-3,7,7- trimethyldecane *arrow_forward(a) Draw all stereoisomers formed by monobromination of the cis and trans isomers of 1,2-dimethylcyclohexane drawn below. (b) How do the products formed from each reactant compare-identical compounds, stereoisomers, or constitutional isomers? cis-1,2-dimethylcyclohexane trans-(1R.2S)-dimethylcyclohexanearrow_forward12a. On the small chair draw the most stable skeletal conformation of rans-1-ethyl-3-isopropylcyclohe. ane, make sure to draw the Hydrogens directly connected to the ring. ( 12b. On the big chair only draw the alkyl groups skeletal and omit the Hydrogens connected to the ring. ( 12a. 12b. draw the correct skeletal structure here draw the cor.ect skeletal structure herearrow_forward
- Show that the addition of each CH2 unit to an alcohol molecule changes the heat of combustion by: 2(C-H)+1(C-C)+1.5(O=O) – [2(C=O)+2(O-H)]arrow_forwardDerive an IUPAC name for the following (cyclo)alkenes. (Do not use cis/trans in your names. Use only the (E)/(Z) designations for double bond stereochemistry. It is not necessary to use italics in writing compound names.) (a) (b) my Xarrow_forwardA The alkyl group (CH3)2CH- is a(n) group. (a) tert-butyl (b) iso-butyl (c) n-butyl (d) sec-butyl (e) iso-propyl B In the box to the right of each molecule, draw a constitutional (structural) isomer of it. Br: NH₂arrow_forward
- We have covered several oxidants that use a multi-valent atom (Cr, Cl, S, or I) as their active species, going from a higher oxidation state before the oxidation to a lower oxidation state after oxidizing the alcohol. Draw the structure of the following atoms, before and after the oxidation of an alcohol to a ketone or aldehyde. How many bonds to oxygen does each atom have before and after the oxidation? (a) the Cr in chromic acid (b) the Cl in sodium hypochlorite (c) the S in the Swern oxidationarrow_forwardWhat is the structure of bicyclo[1.1.0]butan-2-amine?arrow_forwardWrite the structures of two chair conformations of 1-tert-butyl-1-methylcyclohexane. Which conformation is more stable?arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





