
Concept explainers
(a)
Interpretation:
Whether the given potassium chloride and silver nitrate on mixing in aqueous solution, gives a precipitation reaction or not has to be predicted. Also a balanced equation for the precipitation reaction has to be written.
Concept Introduction:
Precipitation reaction is a reaction in which any chemical change in a solution results in giving one or more insoluble products. The insoluble product is called as precipitate.
The compounds that can either form precipitate or it gets soluble can be identified by the information given in figure 1.
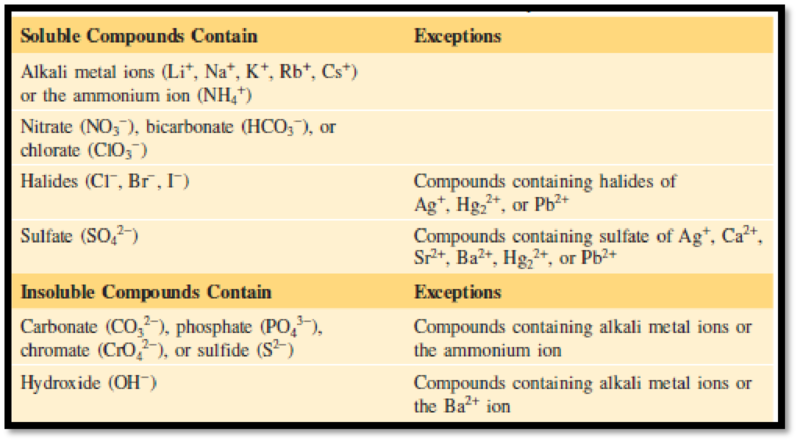
Figure 1
(b)
Interpretation:
Whether the given Potassium carbonate and calcium hydroxide on mixing in aqueous solution, gives a precipitation reaction or not has to be predicted. Also a balanced equation for the precipitation reaction has to be written.
Concept Introduction:
Refer part (a).
(c)
Interpretation:
Whether the given Sodium hydroxide and ammonium chloride on mixing in aqueous solution, gives a precipitation reaction or not has to be predicted. Also a balanced equation for the precipitation reaction has to be written.
Concept Introduction:
Refer part (a).
(d)
Interpretation:
Whether the given Sodium hydroxide and iron
Concept Introduction:
Refer part (a).
Want to see the full answer?
Check out a sample textbook solution
Chapter 4 Solutions
GENERAL,ORGANIC,+BIOCHEMISTRY(LL)-PKG
- Pleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forwardPleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forwardThe Ksp for lead iodide ( Pbl₂) is 1.4 × 10-8. Calculate the solubility of lead iodide in each of the following. a. water Solubility = mol/L b. 0.17 M Pb(NO3)2 Solubility = c. 0.017 M NaI mol/L Solubility = mol/Larrow_forward
- Pleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forwardPleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forwardOnly 100% sure experts solve it correct complete solutions need to get full marks it's my quiz okkkk.take your time but solve full accurate okkk chemistry expert solve itarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





