
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 4.2, Problem 4.4P
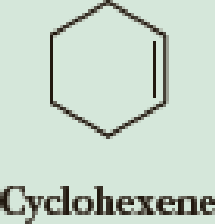
Write an equation to show the proton transfer between each

Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
4-methyl-3-hexanol was prepared by reacting an alkene with either hydroboration-oxidation or oxymercuration-reduction.
Draw the structure of the alkene that was used to prepare the alcohol in highest yield.
• You do not have to consider stereochemistry.
• Indicate the method of preparation by drawing either BH3 (for
. If there is more than one alkene that can be used for a given method, draw all of them.
If either hydroboration-oxidation or oxymercuration-reduction can be used, just give the structures for one method.
Separate structures with + signs from the drop-down menu.
●
981
--
26224
?
ChemDoodle
Y
hydroboration-oxidation), or Hg (for oxymercuration-reduction), in a separate sketcher.
Sn [1
2-methyl-2-hexanol was prepared by reacting an alkene with either hydroboration-oxidation or oxymercuration-reduction.
Draw the structure of the alkene that was used to prepare the alcohol in highest yield.
• You do not have to consider stereochemistry.
• Indicate the method of preparation by drawing either BH3 (for hydroboration-oxidation), or Hg (for oxymercuration-reduction), in a separate sketcher.
• If there is more than one alkene that can be used for a given method, draw all of them.
• If either hydroboration-oxidation or oxymercuration-reduction can be used, just give the structures for one method.
• Separate structures with + signs from the drop-down menu.
*9⁹-85
?
ChemDoodle
Sn [F
1. Consider the solubility and boiling point of the following pair of compounds: n-butyl alcohol and diethyl ether. The boiling points for the compounds are 118 °C and 35 °C respectively. The solubility for both compounds is the same (8g/100g water). Explain this observation for (i) boiling point disparity; (ii) solubility similarity
a. H-bonds form in diethyl ether; n-butyl alcohol forms H-bonds in water
b. H-bonds form in n-butyl alcohol; diethyl ether forms H-bonds in water
c. H-bonds in n-butyl alcohol; Both compounds form H-bonds in water
d. Both compounds form H-bonds; Both compounds form H-bonds in water
2. Account for the bond angle differences between (i) H-C-H (109.5°) in methane and H-S-H (90°); H-C-H (109.5°) and H-O-H (107.5°) in water.
a. The H-S-H has two lone pairs; The H-O-H has two lone pairs
b. The H-S-H has no hybridization at p-orbitals; The H-O-H has two lone pairs
c. The H-S-H has two lone pairs; The H-O-H has no hybridization…
Chapter 4 Solutions
Organic Chemistry
Ch. 4.2 - For each conjugate acid-base pair, identify the...Ch. 4.2 - Write these reactions as proton-transfer...Ch. 4.2 - Following is a structural formula for guanidine,...Ch. 4.2 - Write an equation to show the proton transfer...Ch. 4.3 - For each value of Ka, calculate the corresponding...Ch. 4.4 - Predict the position of equilibrium and calculate...Ch. 4.5 - Calculate Keq for a reaction with G0 = 17.1 kJ/mol...Ch. 4.6 - Acid-Base Equilibria Many factors contribute to...Ch. 4.6 - What is the relative trend in acidity and pKa of...Ch. 4.7 - Write an equation for the reaction between each...
Ch. 4 - For each conjugate acid-base pair, identify the...Ch. 4 - Complete a net ionic equation for each...Ch. 4 - Arrange the compounds in each set in order of...Ch. 4 - Prob. 4.12PCh. 4 - In acetic acid, CH3COOH, the OH hydrogen is more...Ch. 4 - Which has the larger numerical value? (a) The pKa...Ch. 4 - In each pair, select the stronger acid. (a)...Ch. 4 - Arrange the compounds in each set in order of...Ch. 4 - Arrange the compounds in each set in order of...Ch. 4 - If the G for a reaction is 4.5 kcal/mol at 298 K,...Ch. 4 - Calculate the Keq for the following reactions from...Ch. 4 - Prob. 4.20PCh. 4 - Answer true or false to the following statements...Ch. 4 - In each of the following three reaction coordinate...Ch. 4 - The acid-base chemistry reaction of barium...Ch. 4 - Unless under pressure, carbonic acid (H2CO3) in...Ch. 4 - Prob. 4.25PCh. 4 - Acetic acid, CH3COOH, is a weak organic acid, pKa...Ch. 4 - Benzoic acid, C6H5COOH (pKa 4.19), is only...Ch. 4 - Prob. 4.28PCh. 4 - One way to determine the predominant species at...Ch. 4 - Will acetylene react with sodium hydride according...Ch. 4 - Prob. 4.31PCh. 4 - For each equation, label the Lewis acid and the...Ch. 4 - Complete the equation for the reaction between...Ch. 4 - Each of these reactions can be written as a Lewis...Ch. 4 - The sec-butyl cation can react as both a...Ch. 4 - Prob. 4.36APCh. 4 - Prob. 4.37APCh. 4 - Prob. 4.38APCh. 4 - Explain why the hydronium ion, H3O+, is the...Ch. 4 - What is the strongest base that can exist in...Ch. 4 - Prob. 4.42APCh. 4 - Prob. 4.43APCh. 4 - Methyl isocyanate, CH3N=C=O, is used in the...Ch. 4 - Offer an explanation for the following...Ch. 4 - Prob. 4.46APCh. 4 - Alcohols (Chapter 10) are weak organic acids, pKa...Ch. 4 - As we shall see in Chapter 19, hydrogens on a...Ch. 4 - 2,4-Pentanedione is a considerably stronger acid...Ch. 4 - Write an equation for the acid-base reaction...Ch. 4 - Prob. 4.51APCh. 4 - Prob. 4.52APCh. 4 - Prob. 4.53APCh. 4 - Following is a structural formula for imidazole, a...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- When bromine is added to two beakers, one containing phenyl isopropyl ether and the other containing cyclohexene, the bromine color in both beakers disappears. What observation could you make while performing this test that would allow you to distinguish the alkene from the aryl ether?arrow_forwardDraw a structural formula for the alkene you would use to prepare the alcohol shown by hydroboration/oxidation. H3C CH3 CH3CHCCH3 ОН You do not have to consider stereochemistry. • You do not have to explicitly draw H atoms. • In cases where there is more than one answer, just draw one.arrow_forwardWhen cyclopropane is treated with HI, 1-iodopropane is formed. A similar type of reaction does not occur with cyclopentane or cyclohexane. Suggest an explanation for cyclopropane’s reactivity.arrow_forward
- Write an equation to show the proton transfer between each alkene or cycloalkene and HCl. Where two carbocations are possible, show each. (a) CH,CH,CH=CHCH, (b) 2-Pentene Cyclohexenearrow_forwardThis is an organic chemistry question? Identify the major product(s) formed by the following reaction. PLEASE SHOW STEP BY STEP! ALSO PLEASE INCLUDE ALL ELECTRON LONE PAIRS AND CHARGES FOR STRUCTURES IF NECESSARY!arrow_forwardEthanol, C2H5OH, and propane, C3H8, have approximately the same molar mass, yet ethanol has a much higher boiling point. Briefly explain why. Ethanol, C2H5OH, and dimethyl ether, CH3OCH3, have the same molar mass, yet ethanol has a much higher boiling point. Briefly explain why. Write an equation to show the reaction between ethanol, C2H5OH and methyllithium, CH3Li. Draw all non-bonding electrons and show electron flow with curved arrows. 37. Write an equation that shows the reaction between acetic acid (CH3COOH) and triethylamine (CH3CH2)3N. Draw all non-bonding lone electron pairs and show the electron flow with curved arrows.arrow_forward
- Draw a structural formula for the alkene you would use to prepare the alcohol shown by hydroboration/oxidation. CH3 CH3CHCH₂CH₂CH₂OH • You do not have to consider stereochemistry. • You do not have to explicitly draw H atoms. • In cases where there is more than one answer, just draw one.arrow_forward2-methyl-2-hexanol was prepared by reacting an alkene with either hydroboration- oxidation or oxymercuration-reduction. Draw the structure of the alkene that was used to prepare the alcohol in highest yield. You do not have to consider stereochemistry. • Indicate the method of preparation by drawing either BH3 (for hydroboration- oxidation), or Hg (for oxymercuration-reduction), in a separate sketcher. • If there is more than one alkene that can be used for a given method, draw all of them. • If either hydroboration-oxidation or oxymercuration-reduction can be used, just give the structures for one method. Separate structures with + signs from the drop-down menu. ? ChemDoodleⓇ ⒸO [F ▼ [ ] در >arrow_forwardGive at least 3 features in the structure of an alkene.arrow_forward
- Write structural formulas for all ketones with the molecular formula C6H12O and give each its IUPAC name. Which of these ketones are chiral?arrow_forward2) Provide the IUPAC name for the foll pound. 8) Provide the major organic product in the reaction shown below. OH 1. H₂Cr₂O 2. SOCI₂ 3. (CH₂CH₂)₂Cul.iarrow_forwardDraw structural formulas for the alkenes formed by acid-catalyzed dehydration of each alcohol. Where isomeric alkenes are possible, predict which is the major product. Q.) 2-Methyl-2-butanolarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
07 Physical Properties of Organic Compounds; Author: Mindset;https://www.youtube.com/watch?v=UjlSgwq4w6U;License: Standard YouTube License, CC-BY