
Concept explainers
(a)
Interpretation: To determine the type of reaction whether it will be an
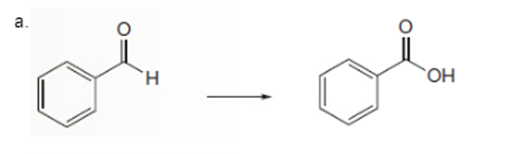
Concept Introduction: Oxidation, which refers to the loss of electrons, is the increase in the oxidation state of its component atoms. When an atom obtains electrons or has its oxidation state reduced, the reduction can occur.
(b)
Interpretation: To determine the type of reaction whether it will be an oxidation or reduction reaction.
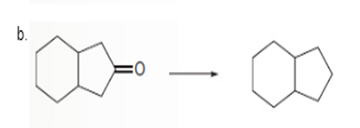
Concept Introduction:
Oxidation, which refers to the loss of electrons, is the increase in the oxidation state of its component atoms. When an atom obtains electrons or has its oxidation state reduced, the reduction can occur.
(c)
Interpretation: To determine the type of reaction whether it will be an oxidation or reduction reaction.
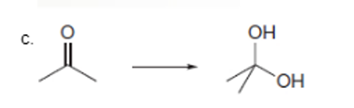
Concept Introduction: Oxidation, which refers to the loss of electrons, is the increase in the oxidation state of its component atoms. When an atom obtains electrons or has its oxidation state reduced, the reduction can occur.
(d)
Interpretation: To determine the type of reaction whether it will be an oxidation or reduction reaction.
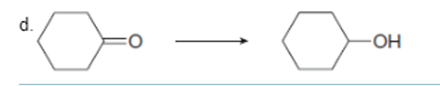
Concept Introduction: Oxidation, which refers to the loss of electrons, is the increase in the oxidation state of its component atoms. When an atom obtains electrons or has its oxidation state reduced, the reduction can occur.
Want to see the full answer?
Check out a sample textbook solution
Chapter 4 Solutions
Organic Chemistry (6th Edition)
- Add missing reactants, reagents and products. Include the stereochemistry if necessary. Unless otherwise stated, assume the reagents are in excess.arrow_forwardThe missing reagent or reaction condition in the following transformation is:arrow_forwardDraw all products, including stereoisomers, in attached reaction.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
