
Concept explainers
(a)
Interpretation:
The dissociation constant of protein and antibody A is to be calculated.
Concept introduction:
The protein is made up of different amino acids. The amino acid is the smallest unit of a protein. The protein may be composed of primary, secondary, tertiary and quarternary structure. The antibody is also a type of protein which is derived by plasma cells and it is used by the immune system of the cells.
Answer to Problem 19P
The dissociation constant of a complex between protein and antibody A is
Explanation of Solution
The dissociation constant is defined as the breaking of larger molecules into its smaller units or it is defined as the ratio of products to the reactants with respect to their stoichiometric number.
The reaction corresponding to the dissociation of PA is given below.
The dissociation constant of the above equation is shown below.
The value of dissociation constant is obtained when half of the complex is dissociated as given below.
This is the concentration of protein which is obtained and the concentration of the complex which is dissociated.
The concentration of antibody A is obtained from the graph shown below.
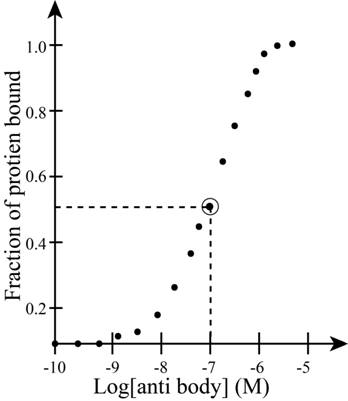
Figure 1
From the graph above.
Substitute values of
Therefore the dissociation constant the complex between protein and antibody A is
(b)
Interpretation:
The dissociation constant of protein and antibody B is to be calculated.
Concept introduction:
The protein is made up of different amino acids. The amino acid is the smallest unit of a protein. The protein may be composed of primary, secondary, tertiary and quarternary structure. The antibody is also a type of protein which is derived by plasma cells and it is used by the immune system of the cells.
Answer to Problem 19P
The dissociation constant of a complex between protein and antibody B is
Explanation of Solution
The dissociation constant is defined as the breaking of larger molecules into its smaller units or it is defined as the ratio of products to the reactants with respect to their stoichiometric number.
Consider a reaction given below.
The dissociation constant of the above equation is shown below.
The value of dissociation constant is obtained when half of the complex is dissociated as given below.
This is the concentration of protein which is obtained and the concentration of the complex which is dissociated.
The concentration of antibody B is obtained from the graph shown below.
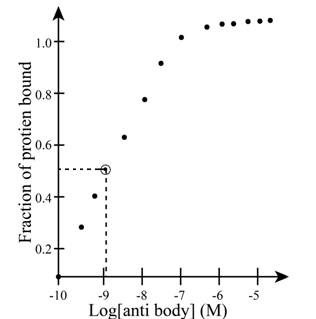
Figure 2
From the graph above, the concentration of the antibody is calculated as follows:
Substitute values of
Therefore the dissociation constant the complex between protein and antibody B is
On comparing, it is found that antibody A and B are identical except for one single amino acid. The reason for the generation of antibody B is through gene mutation which permanentlychanges the DNA sequence of a species. It may be hereditary or acquired mutation. Since in this case only one amino acid changes.
Therefore, the mutation is a point mutationthat generated antibody B.
Want to see more full solutions like this?
- Docking and Membrane Fusion. Q-8a. Choose from the terms below to Fill-in the Blanks. [All terms are used. Some terms are used more than once] Rab, SNARE, v- SNARE, t-SNARE, Tethering a. Identification of a vesicle to be docked depends on a diverse family of monomeric GTPases called proteins. First, a filamentous protein on a target membrane binds to a protein on the surface of a vesicle. This interaction allows the vesicle to dock on its particular target membrane. A on the vesicle then binds to a complementary_ on the target membrane. Whereas and proteins provide the initial recognition between a vesicle and its target membrane, complementary appropriate target membranes. Together, the proteins ensure that transport vesicles dock at their proteins catalyze the final fusion of the two membranes by squeezing out water making fusion more energetically favorable. b. What does the acronym SNARE stand for? c. Membrane fusion the rate limiting step of vesicular transport. Why? (What makes…arrow_forwardBONUS QUESTION! In neurons, the proteolytic enzyme y-secretase produces the Aß amyloid peptides shown below. The AB40 peptide is thought to play a protective role in the neuron. However, the AB42 peptide appears to be toxic since it is found in the amyloid plaques that cause Alzheimer's Disease (AD). AB40 and AB42 are identical, except that AB42 contains two extra amino acid residues (shown in red) at the C-terminal end. Based on your knowledge of amino acids and proteins, which of the following factors is most likely to explain the greater plaque-forming activity of AB42 compared to AB40? Sequence of Aß40: DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVV Sequence of AB42: DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVVIA O The greater length of AB42 makes it more likely to aggregate and form plaques. O AB42 has a lower pl than AB40, which makes it more likely to aggregate at physiological pH. O AB42 is more hydrophobic than AB40, which makes it harder to clear from the cell and thus more likely to…arrow_forwardIn Multi-Column Purification of rGFP. What happens to the protein amount, protein purity, and/or specific activity of a purification fraction if one of the three is changed? (i.e. understand the relationship between the three.)arrow_forward
- In SDS-PAGE. What chemical is used to ensure that all protein molecules are coated with a negative charge? IPTG. β-mercaptoethanol. X-Gal. SDS.arrow_forward. What physiological effect would you predict from a mutation that replaced with serine the cysteine in the constant part of the immunoglobulin light chain that is involved in disulfide-bond for- mation with the heavy chain? ,arrow_forwardPlease help me with this question. More than one answer may be correct. THe graph relating to the information is included below. Dilated cardiomyopathy (DCM) is a condition in which the wall of the heart, starting with the left ventricle, stretches and thins. This prevents the heart from pumping effectively and can be life threatening. The above graph shows mRNA expression (left pane) and protein expression (right pane) for three molecular components of cardiomyocytes (GJA3 is the gene that encodes for Connexin 46, DSP is the gene that encodes for Desmoplakin, and CTNNA3 is the gene that encodes for Catenin α-3) between DCM cells and healthy cardiomyocytes (CNT). Based on the above figure, which of the following issues are hearts with dilated cardiomyopathy likely to suffer? Options: impaired electrical conduction between cells impaired cell-cell communication poor cell-cell adhesion decreased energetic output from mitochondria impaired ability to pass mitochondria between cellsarrow_forward
- Initiation. Bacterial protein synthesis is initiated by: a. S-adenosylmethionyl tRNA b. Methionyl TRNA c. N-formylmethionyl tRNA d. N10-formyltetrahydrofolateN"-formyltetrahydrofolate †RNA „N10arrow_forwardBiomedical Engineering: An equilibrium dialysis experiment was performed to characterize the binding affinity of a mouse IgG or human IgG antibody for an antigen, GAD65. (The association constant for the monoclonal mouse IgG is 4.75 x 10^8 M-1 and for a human IgG is 1.3 x 10^10 M-1) a- calculate the dissociation constant for each antibody and explain which antibody has a higher binding affinity for GAD65? b-calculate the fraction of free Ab sites for each antibody for a For a GAD65 concentration of 0.7 nMarrow_forwardPlease help me with this question. How many amino acid residues are in the heavy and light chains of the Fab fragment, and how many amino acid residues are in lysozyme?arrow_forward
- cancer. Describe how experiments can be performed and used to determine the accuracy, sensitivity and specificity of the device. Using your own hypothetical values, calculate the accuracy, sensitivity and specificity of the device. Is the device suitable for screening for breast cancer, based on your calculated valuesarrow_forwardAlpha polypeptide (ADH1A). Give a detailed description of its role in the disease. Describe the impact of the disease on society.Describe a way in which your gene can be manipulated to treat the disease. Assume you can make any changes to the protein product and describe specifically how it will affect its interaction with other molecules.arrow_forwardPlease help me with this question. More than one answer may be correct. THe graph relating to the information is included below. In the two figures, a wild-type plant and a plant with a atg5 knockout, which prevents autophagy within the cell, are compared. The first figure shows the number of peroxisomes per micrograph image taken of the plant's leaves. The bottom figure shows a comparison of 3-week seedlings of the wild-type plant and the atg5 plant at normal CO2 levels (360 ppm) and elevated CO2 (2000 ppm) Which of the following are true. Question 20 options: the atg5 knockout plants are more able to deal with photorespiration the atg5 knockout plants are less able to deal with photorespiration the inability to recycle peroxisomes through autophagy impairs a plants ability to deal with photorespiration Elevated CO2 overcomes the photorespiration impairment in atg5 plants Elevated CO2 overcomes the photorespiration impairment in wild-type plantsarrow_forward
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON





