
Organic Chemistry (6th Edition)
6th Edition
ISBN: 9781260119107
Author: Janice Gorzynski Smith
Publisher: McGraw Hill Education
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 3.4, Problem 18P
(a) Label the hydrophobic and hydrophilic portions of each molecule. (b) At which sites can C hydrogen bond to another molecule like itself? (c) At which sites can D hydrogen bond to water?
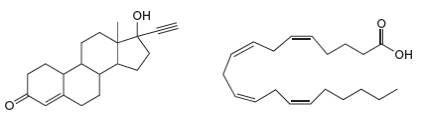
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
5. Build another hexane molecule and place it alongside
the first one you built.
(f) What types of intermolecular forces help hold hexane
molecules to each other?
(g) What aspect of the molecules' geometry makes these
bonds effective?
(a) Which of these compounds below would you expectto have the highest boiling point? (b) Which of thesecompounds is the most oxidized? (c) Which of these compounds,if any, is an ether? (d) Which of these compounds,if any, is an ester? (e) Which of these compounds, if any, isa ketone?
Consider lisinopril, a drug used primarily in the treatment of high blood pressure, heart failure, and after heart attacks.
(a) Name the various functional groups in lisinopril.
(b) What intermolecular forces are expected to exist between molecules of lisinopril in close proximity to one another (Section 5.7)?
Chapter 3 Solutions
Organic Chemistry (6th Edition)
Ch. 3.1 - Prob. 1PCh. 3.2 - (a) Classify the carbon atoms in each compound as...Ch. 3.2 - Problem 3.3 Classify a carbon atom by the number...Ch. 3.2 - Classify each alkyl halide and alcohol as , or...Ch. 3.2 - Prob. 5PCh. 3.2 - Prob. 6PCh. 3.2 - Draw the structure of a compound of molecular...Ch. 3.2 - Prob. 8PCh. 3.2 - Prob. 9PCh. 3.2 - Draw the structure of a compound fitting each...
Ch. 3.4 - Predict which compound in each pair has the higher...Ch. 3.4 - Prob. 17PCh. 3.4 - a Label the hydrophobic and hydrophilic portions...Ch. 3.5 - Prob. 21PCh. 3 - 3.29
Identify the functional groups in the...Ch. 3 - Prob. 32PCh. 3 - 3.31 For each alkane: (a) classify each carbon...Ch. 3 - 3.32 Identify the functional groups in each...Ch. 3 - 3.33 Identify each functional group located in the...Ch. 3 - 3.34 (a)Identify the functional groups in...Ch. 3 - Draw seven constitutional isomers with molecular...Ch. 3 - Prob. 38PCh. 3 - Prob. 39PCh. 3 - Prob. 40PCh. 3 - Intramolecular force of attraction are often...Ch. 3 - 3.40 (a) Draw four compounds with molecular...Ch. 3 - 3.41 Rank the compounds in each group in order of...Ch. 3 - Explain why CH3CH2NHCH3 has higher boiling point...Ch. 3 - Prob. 45PCh. 3 - 3.44 Rank the following compounds in order of...Ch. 3 - Prob. 47PCh. 3 - 3.50 Predict the solubility of each of the...Ch. 3 - Prob. 52PCh. 3 - Prob. 53PCh. 3 - 3.53 THC is the active component in marijuana, and...Ch. 3 - Prob. 55PCh. 3 - Prob. 56PCh. 3 - 3.60 Quinapril (trade name Accupril) is a drug...Ch. 3 - 3.61 Answer each question about oxycodone, a...Ch. 3 - Prob. 65PCh. 3 - Prob. 66PCh. 3 - 3.64 Explain why A is less water soluble than B,...Ch. 3 - 3.65 Recall from section 1.10B that there is...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- (a) At which sites can C hydrogen bond to another molecule like itself? (b) At which sites can D hydrogen bond to water?arrow_forwardRank the attached compounds in order of increasing boiling point. Which compound is the most water soluble? Which compound is the least water soluble?arrow_forwardThe fact that sweet-tasting carbohydrates like table sugar are also high in calories has prompted the development of sweet, low-calorie alternatives. (a) Identify the functional groups in aspartame, the artificial sweetener in Equal. (b) Label all of the sites that can hydrogen bond to the oxygen atom of water. (c) Label all of the sites that can hydrogen bond to a hydrogen atom of water.arrow_forward
- The fact that sweet-tasting carbohydrates like table sugar are also high in calories has prompted the development of sweet, low-calorie alternatives. (a) Identify the functional groups in aspartame, the artificial sweetener in Equal. (b) Label all of the sites that can hydrogen bond to the oxygen atom of water.arrow_forwardCarbon bonds to many elements other than itself.(a) Name six elements that commonly bond to carbon in organiccompounds.(b) Which of these elements are heteroatoms?(c) Which of these elements are more electronegative than car-bon? Less electronegative?(d) How does bonding of carbon to heteroatoms increase thenumber of organic compounds?arrow_forward(a) Which of the following compounds, if any, is an ether? (b) Which compound, If any, is an alcohol? (c) Which com- pound, if any, would produce a basic solution if dissolved in water? (Assume solubility is not a problem). (d) Which compound, if any, is a ketone? (e) Which compound, if any, is an aldehyde? () Н,С—CH;—он Н (ii) H;C-Ñ-CH,CH=CH2 (ii) o (iv) (v) CH;CH,CH,CH2CHO (vi) CH3C=CCH,COOHarrow_forward
- Indinavir (trade name Crixivan) is a drug used to treat HIV. (a) At which sites can indinavir hydrogen bond to another molecule like itself? (b) At which sites can indinavir hydrogen bond to water? H. IN. HO N. N' он indinavirarrow_forwardFor the following, you must draw an appropriate structure that has the chemical formula C5H,NO with the indicated functional group(s) and/or property. In each case, identify any other functional groups in the molecule you draw, that were not indicated in the question. You may use condensed dash or bond-line structure to draw your molecules. a) An aldehyde b) A cyclic ether c) An acyclic amide that cannot form hydrogen bonds with itselfarrow_forward(a) Identify the functional groups in salinosporamide A, an anticancer agent isolated from marine sediment, (b) Classify each alcohol, alkyl halide, amide, and amine as 1°, 2°, or 3°.arrow_forward
- Sphingomyelins, a group of lipids that resemble the membrane phospholipids discussed in Section 3.7, are a major component of the myelin sheath, the insulating layer that surrounds a nerve fiber. (A) What functional groups are present in sphingomyelin X? (B) Classify any alcohol, amine, and amide as 1, 2, or 3. (C) Label the polar head and non polar tails of X.arrow_forward1. Why is the compound caffeine made? Answer Nd discuss it comprehesively.arrow_forwardName the type of organic compound from the following de-scription of its functional group: (a) N-containing group withsingle and double bonds; (b) group that is not polar and has adouble bond; (c) polar group that has a double bond and cannotbe at the end of a C chain; (d) group that has only single bondsand is basic in water.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning
07 Physical Properties of Organic Compounds; Author: Mindset;https://www.youtube.com/watch?v=UjlSgwq4w6U;License: Standard YouTube License, CC-BY