
Organic Chemistry (6th Edition)
6th Edition
ISBN: 9781260119107
Author: Janice Gorzynski Smith
Publisher: McGraw Hill Education
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 3.2, Problem 4P
Classify each
a. 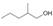 b.
b. 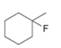 c.
c. 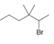 d.
d. 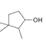
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Which alkyl halide has the highest boiling point? A. CH3BrB. CH3FC. CH3ClD. CH3l
Glycerol contains:
a. oxygens which are each bonded to two alkyl groups
b. oxygens single-bonded to primary and secondary carbons
c. Oxygens double-bonded to carbon, with alkyls on both sides
d. Oxygens double-bonded to carbon, with alkyls on one side only
e. Oxygens double-bonded to carbon, with an alkyl on one side and an --OH on the other side
1. Which functional group is found in aldehydes?
a. CHO
b. -CH;OH
c. -COOH
d. RCOR'
2. Which functional group is found in ketones?
a. -CHO
CH:OH
c. -COOH
RCOR'
3. Which structural feature is common to aldehydes and ketones?
a, an oxygen atom bonded to both a carbon atom and a hydrogen atom
b. an oxygen atom bonded to two carbon atoms
c. an oxygen atom double bonded to a carbon atom
d. two oxygen atoms bonded to the same carbon atom
4. What is the IUPAC name for the following compound?
a. I-pentaldehyde b. l-pentanal
c. pentanal
d. pentanealdehyde
Cetoa of Fi
Chapter 3 Solutions
Organic Chemistry (6th Edition)
Ch. 3.1 - Prob. 1PCh. 3.2 - (a) Classify the carbon atoms in each compound as...Ch. 3.2 - Problem 3.3 Classify a carbon atom by the number...Ch. 3.2 - Classify each alkyl halide and alcohol as , or...Ch. 3.2 - Prob. 5PCh. 3.2 - Prob. 6PCh. 3.2 - Draw the structure of a compound of molecular...Ch. 3.2 - Prob. 8PCh. 3.2 - Prob. 9PCh. 3.2 - Draw the structure of a compound fitting each...
Ch. 3.4 - Predict which compound in each pair has the higher...Ch. 3.4 - Prob. 17PCh. 3.4 - a Label the hydrophobic and hydrophilic portions...Ch. 3.5 - Prob. 21PCh. 3 - 3.29
Identify the functional groups in the...Ch. 3 - Prob. 32PCh. 3 - 3.31 For each alkane: (a) classify each carbon...Ch. 3 - 3.32 Identify the functional groups in each...Ch. 3 - 3.33 Identify each functional group located in the...Ch. 3 - 3.34 (a)Identify the functional groups in...Ch. 3 - Draw seven constitutional isomers with molecular...Ch. 3 - Prob. 38PCh. 3 - Prob. 39PCh. 3 - Prob. 40PCh. 3 - Intramolecular force of attraction are often...Ch. 3 - 3.40 (a) Draw four compounds with molecular...Ch. 3 - 3.41 Rank the compounds in each group in order of...Ch. 3 - Explain why CH3CH2NHCH3 has higher boiling point...Ch. 3 - Prob. 45PCh. 3 - 3.44 Rank the following compounds in order of...Ch. 3 - Prob. 47PCh. 3 - 3.50 Predict the solubility of each of the...Ch. 3 - Prob. 52PCh. 3 - Prob. 53PCh. 3 - 3.53 THC is the active component in marijuana, and...Ch. 3 - Prob. 55PCh. 3 - Prob. 56PCh. 3 - 3.60 Quinapril (trade name Accupril) is a drug...Ch. 3 - 3.61 Answer each question about oxycodone, a...Ch. 3 - Prob. 65PCh. 3 - Prob. 66PCh. 3 - 3.64 Explain why A is less water soluble than B,...Ch. 3 - 3.65 Recall from section 1.10B that there is...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Name each compound. A. B. C. CH₂CH3 CH₂CH3 a & CI CI CI Br. ď OH CI compound A name: compound B name: compound C name:arrow_forward18. Ketone reduction Dicyclohexyl ketone Reduce the ketone. 1. NaBH4, ethanol 2. H3O+ H OH Dicyclohexylmethanol (88%) (a 2° alcohol)arrow_forward3) Identify the compound with the lowest boiling point. A. CH3CH2CH2OH B. CH:CH(OH)CH3 C. CH3OCH2CH3 D. CH3CH2CH2CH2OH E. CH3CH2OCH2CH3arrow_forward
- 1. Differentiate Ethyl alcohol from methyl alcohol. 2. Compare aldehydes and ketones as to (Use acetaldehyde and acetone as examples). A. Reaction with cone. NaOH, heated B. Reaction with Tollen’s reagentarrow_forward1. Write the IUPAC name of each compound. a. Br b. C.arrow_forward16. Identify each compound as an a cohol, a phenol, or an ether. Classify any alcohols as primary (1"), secondary (2), or tertiary (3"). a. CH,CH,CH,OH CH,CHCH, b. CHO C. CH CHOCH, CH, d.arrow_forward
- 4. What alcohol is formed when each compound is treated with NaBH4 in MEOH? a. NaBH MeOH H3CH,CH,C H. NABH4 b. MEOH C. NaBH MEOH Section: Row: 12 Column: 4 Words: 114 O Spell Check O Local backup on Page Num: 1 Page: 1/4 1/1 MacBook Pro 411 F6 F4 esc FI F2 & %23 24 3 4. 6. R T tab Caarrow_forwardName each compound depicted in the ball-and-stick models. a. C.arrow_forwardClassify each alkyl halide as 1°, 2°, or 3°. CH3 c. CHg-C-CHCH3 ČH3 ČI CH;CH2CH,CH,CH2-Br b. d. a.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning
07 Physical Properties of Organic Compounds; Author: Mindset;https://www.youtube.com/watch?v=UjlSgwq4w6U;License: Standard YouTube License, CC-BY