
Biochemistry: Concepts and Connections
1st Edition
ISBN: 9780321839923
Author: Dean R. Appling, Spencer J. Anthony-Cahill, Christopher K. Mathews
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 3, Problem 6P
In another key reaction in glycolysis, dihydroxyacetone phosphate (DHAP) is isomerized into glyceraldehyde-3-phosphate (GAP);
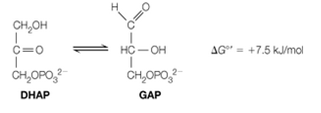
Because
a. Calculate the equilibrium constant and the equilibrium fraction of GAP from the above, at 37 oC.
b. In the cell, depletion of GAP makes the reaction proceed. What will
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
In another key reaction in glycolysis, dihydroxyacetone phosphate (DHAP)is isomerized into glyceraldehyde-3-phosphate (GAP): Because ΔG°′ is positive, the equilibrium lies to the left.(a) Calculate the equilibrium constant and the equilibrium fraction of GAPfrom the above, at 37 °C.(b) In the cell, depletion of GAP makes the reaction proceed. What will ΔGbe if the concentration of GAP is always kept at 1/100 of the concentrationof DHAP?
Decylic acid is a saturated fatty
acid that occurs naturally in
coconut oil and palm kernel oil.
Calculate the net ATP yield when
decylic acid undergoes complete
B oxidation. The formula of
decylic acid is shown below:
(Given: The oxidation of one
NADH yields 2.5 ATP; the
oxidation of one FADH2 yields
1.5 ATP; and the oxidation of
one acetyl CoA yields 10 ATP.)
O 50 ATP
O 52 ATP
66 ATP
OH
O 64 ATP
Consider a series of experiments in which 32P-labeled inorganic phosphate were introduced to erythrocytes
(red blood cells) undergoing glycolysis. Would you expect to see the labeled phosphate in any glycolytic
intermediates? If so, which and at what relative concentrations?
Chapter 3 Solutions
Biochemistry: Concepts and Connections
Ch. 3 - Prob. 1PCh. 3 - Given the following reactions and their...Ch. 3 - The decomposition of crystalline N2O5...Ch. 3 - The oxidation of glucose to CO2 and water is a...Ch. 3 - Prob. 5PCh. 3 - In another key reaction in glycolysis,...Ch. 3 - Assume that some protein molecule, in its folded...Ch. 3 - When a hydrophobic substance like a hydrocarbon is...Ch. 3 - It is observed that as temperature is increased,...Ch. 3 - Suppose a reaction has Ho and So values...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- Coupled reactions occur where a nonspontaneous reaction is enabled by coupling it to a spontaneous reaction. This approach is common in biological settings. Determine if ATP could be generated by this biochemical reaction. You have calculated that cell potential is +0.637V. An example of a coupled reaction is the first step of glycolysis, the phosphorylation of glucose to form glucose-6-phosphate shown below. The net ∆Gº for this reaction isarrow_forwardThe enzyme phosphoglucomutase catalyzes the conversion of glucose 1-phosphate to glucose 6-phosphate. After the reactants and products were mixed and allowed to reach equilibrium at 25°C, the concentration of glucose 1-phosphate was 4.5 mM and that of glucose 6-phosphate was 86 mM. Calculate Keq' and AG for this reaction. The reaction coordinate diagram for an enzyme-catalyzed reaction is shown below. How many transition states and intermediates are in the reaction? Is the reaction thermodynamically favorable? Which step is the rate-determining step of the reaction? G Reaction coordinatearrow_forwardCoupled reactions occur where a nonspontaneous reaction is enabled by coupling it to a spontaneous reaction. This approach is common in biological settings. Determine if ATP could be generated by this biochemical reaction. You have calculated that cell potential is +0.637V. An example of a coupled reaction is the first step of glycolysis, the phosphorylation of glucose to form glucose-6-phosphate shown below. kJ/mol The net AG° for this reaction is 1 2 3 н он H. H- H- H H H H 4 6. H. glucose phosphate anion glucose-6-phosphate AG = +14.0 kJ/mol 7 8 9. АТР ADP phosphate anion AG = -30.5 kJ/mol +/- LOarrow_forward
- The enzyme aldolase catalyzes the reaction shown in the glycolytic pathway: Fructose 1,6-bisphosphate dihydroxyacetone phosphate + glyceraldehyde 3-phosphate The AG" for the reaction is +23.8 kJ mol¯¹ (+5.7 kcal mol−¹), whereas the AG in the cell is −1.3 kJ mol¯¹ (−0.3 kcal mol¯¹). Calculate the ratio of products to reactants under standard (equilibrium) conditions at 37°C. [products] [reactants] 7 x10-5 [products] [reactants] Incorrect Aldolase ===== Calculate the ratio of products to reactants under intracellular conditions at 37°C. 4 ×10-5 Incorrect Complete the statement using your results. under standard conditions under intracellular conditions A reaction that is endergonic under standard conditions can be converted into an exergonic reaction by maintaining the ratio of products to reactants below the equilibrium value.arrow_forwardOne of the examples that we have used to illustrate the concept of equilibrium is the isomerization of glucose-6-phosphate (G6P) to fructose-6-phosphate (F6P), which is the second step in g ycolysis. Draw a graph to show how the reaction Gibbs energy varies with the fraction fof F6P in solution.Label the regions of the graph that correspond to the formation of F6P and G6P being spontaneous, respectively.arrow_forwardIn the third step of glycolysis, the given reactions are coupled. reaction 1: fructose-6-phosphate + Pi ⟶ fructose-1,6-bisphosphate + H2O (Δ? = −28 kJ/mol) reaction 2: ATP + H2O ⟶ ADP + Pi (Δ? = +13.8 kJ/mol) Calculate the overall ΔG (kJ/mol) for the coupled reaction.arrow_forward
- The following incorrect description of the HMS path is () A, 6-P-glucose can be converted into pentose phosphate through this pathway B. Four-carbon and seven-carbon sugars can be provided through this route C. For every mole of carbon dioxide produced when 6-P-glucose is converted to pentose phosphate, it also produces 1 mole of NADPH D, 6-P-glucose is decomposed in this way without consumption of ATP Among the following enzyme-catalyzed reactions that can generate substrate level phosphorylation to generate GTP are () A, hexokinase B, enolase C, succinate thiokinase D, succinate dehydrogenase The limiting factor of fatty acid synthesis in cell fluid is (). A, condensation enzyme B, hydration enzyme C, lipoacyl group transferase D, acetyl-CoA carboxylase Which of the following amino acids is an essential amino acid? () A.Thr B.Lys C.Met D.Argarrow_forwardThe protein catalase is an enzyme that catalyzes the decomposition of hydrogen peroxide:2 H2O2 (aq) → 2 H2O (l) + O2 (g)and has a Michaelis-Menten constant of 25 × 10-3 mol·dm-3 and a turnover number of 4.0×107s-1.The total enzyme concentration is 0.016×10-6 mol·dm-3 and the initial substrate concentration is4.32×10-6 mol·dm-3 Calculate the maximum reaction rate (????) for this enzyme, and the initial rateof this reaction. Note that catalase has a single active site.arrow_forwardConsider the reaction: malate + NAD+ → oxaloacetate + NADH + H+. Calculate ΔE°’ for the reaction.arrow_forward
- In order for fatty acids to enter the mitochondria to be catabolized via β-oxidation, they must first be reacted with coenzyme A (reaction ①). Use the equations below to answer parts a-c. ① Fatty Acid + CoA → Fatty Acid—CoA ΔG = ??? ② ATP → AMP + PPi ΔG = ─45.6 kJ/mol ③ PPi → 2 Pi ΔG = ─19.2 kJ/mol ④ Fatty Acid + ATP + CoA → FA—CoA + AMP + 2 Pi ΔG = ─34 kJ/mol a) Calculate ΔG for reaction 1. b) Suppose the formation of the fatty acid--CoA proceeded via the reaction of ATP to ADP instead of AMP. Calculate ΔG for reaction 5. ① Fatty Acid + CoA → Fatty Acid—CoA ΔG = from part a ② ATP → ADP + Pi ΔG = ─30.5 kJ/mol ⑤ Fatty Acid + ATP + CoA → FA—CoA + ADP + Pi ΔG = ??? kJ c) So why does the…arrow_forwardWhen grown anaerobically on glucose, yeast (S. cerevisiae) converts pyruvate to acetaldehyde, then reduces acetaldehyde to Pethanol using electrons from NADH. Write the chemical equation for the reaction that reduces acetaldehyde (CH3CHO) to ethanol (CH3CH2OH). The table provides the standard reduction potential, E', of the relevant half-reactions. Half-reaction Acetaldehyde + 2 H+ + 2e¯ → ethanol NAD+ + 2H+ + 2e¯ → NADH + H+ E'° (V) -.197 -.320 Calculate the equilibrium constant, K'eq, at 25.0 °C for the reaction that reduces acetaldehyde to ethanol. K'e ×10 = eqarrow_forwardConsider the following reaction occurring at 298 K: OH CH₂OH OH -0. OH OH +ATP k₁ k₂ 0- OCH, OH OH 0 OH OH Glucose Glucose 6-phosphate Hexokinase is an enzyme that catalyzes this reaction. When the hexokinase is present the rate of k₁ is 1.3 X10-3 M-15-1 Calculate the activation energy for the hexokinase catalyzed forward reaction.. +ADParrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON
Mitochondrial mutations; Author: Useful Genetics;https://www.youtube.com/watch?v=GvgXe-3RJeU;License: CC-BY