
(a)
Interpretation:
The more acidic compound from each given pair of compounds should be identified in each of the given anions.
Concept introduction:
Acidic strength in the molecule depends on the stability of the Conjugate anion.
The anion involving in resonance in a molecule will have greater stability when compared to molecules having single anion. In resonance the delocalization of anion would take place.
In a molecule where the anion is carried by a more electronegative atom will show more stability. The greater electronegativity makes anion closer to the nucleus hence more stabilized. If the negative charge located atom is larger enough to hold the negative charge, then anion attains more stability.
The carbanion stability varies with the percentage of ‘s’character in its hybridization state. Since the s-orbital is closer to nucleus than p-orbital, the hybridized orbital having more‘s’ character will have more stability to accommodate anion (due to high nuclear charge). The order is 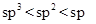 .
.
Inductive effect in a compound will affect the anionic stability. +I groups in the compound stabilizes the negative charge of anion.
To find: the more acidic compound from each given pair of compounds.
(b)
Interpretation:
The more acidic compound from each given pair of compounds should be identified in each of the given anions.
Concept introduction:
Acidic strength in the molecule depends on the stability of the Conjugate anion.
The anion involving in resonance in a molecule will have greater stability when compared to molecules having single anion. In resonance the delocalization of anion would take place.
In a molecule where the anion is carried by a more electronegative atom will show more stability. The greater electronegativity makes anion closer to the nucleus hence more stabilized. If the negative charge located atom is larger enough to hold the negative charge, then anion attains more stability.
The carbanion stability varies with the percentage of ‘s’character in its hybridization state. Since the s-orbital is closer to nucleus than p-orbital, the hybridized orbital having more‘s’ character will have more stability to accommodate anion (due to high nuclear charge). The order is 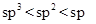 .
.
Inductive effect in a compound will affect the anionic stability. +I groups in the compound stabilizes the negative charge of anion.
To find: the more acidic compound from each given pair of compounds.
(c)
Interpretation:
The more acidic compound from each given pair of compounds should be identified in each of the given anions.
Concept introduction:
Acidic strength in the molecule depends on the stability of the Conjugate anion.
The anion involving in resonance in a molecule will have greater stability when compared to molecules having single anion. In resonance the delocalization of anion would take place.
In a molecule where the anion is carried by a more electronegative atom will show more stability. The greater electronegativity makes anion closer to the nucleus hence more stabilized. If the negative charge located atom is larger enough to hold the negative charge, then anion attains more stability.
The carbanion stability varies with the percentage of ‘s’character in its hybridization state. Since the s-orbital is closer to nucleus than p-orbital, the hybridized orbital having more‘s’ character will have more stability to accommodate anion (due to high nuclear charge). The order is 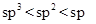 .
.
Inductive effect in a compound will affect the anionic stability. +I groups in the compound stabilizes the negative charge of anion.
To find: the more acidic compound from each given pair of compounds.
(d)
Interpretation:
The more acidic compound from each given pair of compounds should be identified in each of the given anions.
Concept introduction:
Acidic strength in the molecule depends on the stability of the Conjugate anion.
The anion involving in resonance in a molecule will have greater stability when compared to molecules having single anion. In resonance the delocalization of anion would take place.
In a molecule where the anion is carried by a more electronegative atom will show more stability. The greater electronegativity makes anion closer to the nucleus hence more stabilized. If the negative charge located atom is larger enough to hold the negative charge, then anion attains more stability.
The carbanion stability varies with the percentage of ‘s’character in its hybridization state. Since the s-orbital is closer to nucleus than p-orbital, the hybridized orbital having more‘s’ character will have more stability to accommodate anion (due to high nuclear charge). The order is 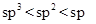 .
.
Inductive effect in a compound will affect the anionic stability. +I groups in the compound stabilizes the negative charge of anion.
To find: the more acidic compound from each given pair of compounds.
(e)
Interpretation:
The more acidic compound from each given pair of compounds should be identified in each of the given anions.
Concept introduction:
Acidic strength in the molecule depends on the stability of the Conjugate anion.
The anion involving in resonance in a molecule will have greater stability when compared to molecules having single anion. In resonance the delocalization of anion would take place.
In a molecule where the anion is carried by a more electronegative atom will show more stability. The greater electronegativity makes anion closer to the nucleus hence more stabilized. If the negative charge located atom is larger enough to hold the negative charge, then anion attains more stability.
The carbanion stability varies with the percentage of ‘s’character in its hybridization state. Since the s-orbital is closer to nucleus than p-orbital, the hybridized orbital having more‘s’ character will have more stability to accommodate anion (due to high nuclear charge). The order is 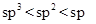 .
.
Inductive effect in a compound will affect the anionic stability. +I groups in the compound stabilizes the negative charge of anion.
To find: the more acidic compound from each given pair of compounds.
(f)
Interpretation:
The more acidic compound from each given pair of compounds should be identified in each of the given anions.
Concept introduction:
Acidic strength in the molecule depends on the stability of the Conjugate anion.
The anion involving in resonance in a molecule will have greater stability when compared to molecules having single anion. In resonance the delocalization of anion would take place.
In a molecule where the anion is carried by a more electronegative atom will show more stability. The greater electronegativity makes anion closer to the nucleus hence more stabilized. If the negative charge located atom is larger enough to hold the negative charge, then anion attains more stability.
The carbanion stability varies with the percentage of ‘s’character in its hybridization state. Since the s-orbital is closer to nucleus than p-orbital, the hybridized orbital having more‘s’ character will have more stability to accommodate anion (due to high nuclear charge). The order is 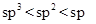 .
.
Inductive effect in a compound will affect the anionic stability. +I groups in the compound stabilizes the negative charge of anion.
To find: the more acidic compound from each given pair of compounds.
(g)
Interpretation:
The more acidic compound from each given pair of compounds should be identified in each of the given anions.
Concept introduction:
Acidic strength in the molecule depends on the stability of the Conjugate anion.
The anion involving in resonance in a molecule will have greater stability when compared to molecules having single anion. In resonance the delocalization of anion would take place.
In a molecule where the anion is carried by a more electronegative atom will show more stability. The greater electronegativity makes anion closer to the nucleus hence more stabilized. If the negative charge located atom is larger enough to hold the negative charge, then anion attains more stability.
The carbanion stability varies with the percentage of ‘s’character in its hybridization state. Since the s-orbital is closer to nucleus than p-orbital, the hybridized orbital having more‘s’ character will have more stability to accommodate anion (due to high nuclear charge). The order is 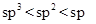 .
.
Inductive effect in a compound will affect the anionic stability. +I groups in the compound stabilizes the negative charge of anion.
To find: the more acidic compound from each given pair of compounds.
(h)
Interpretation:
The more acidic compound from each given pair of compounds should be identified in each of the given anions.
Concept introduction:
Acidic strength in the molecule depends on the stability of the Conjugate anion.
The anion involving in resonance in a molecule will have greater stability when compared to molecules having single anion. In resonance the delocalization of anion would take place.
In a molecule where the anion is carried by a more electronegative atom will show more stability. The greater electronegativity makes anion closer to the nucleus hence more stabilized. If the negative charge located atom is larger enough to hold the negative charge, then anion attains more stability.
The carbanion stability varies with the percentage of ‘s’character in its hybridization state. Since the s-orbital is closer to nucleus than p-orbital, the hybridized orbital having more‘s’ character will have more stability to accommodate anion (due to high nuclear charge). The order is 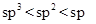 .
.
Inductive effect in a compound will affect the anionic stability. +I groups in the compound stabilizes the negative charge of anion.
To find: the more acidic compound from each given pair of compounds.
Trending nowThis is a popular solution!

Chapter 3 Solutions
Organic Chemistry
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





