
Concept explainers
Interpretation:
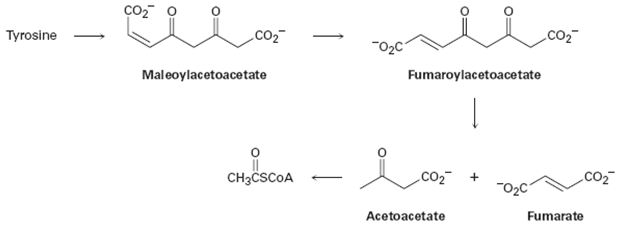
The amino acid tyrosine is biologically degraded by a series of steps that include the following transformations:
Concept introduction:
α-amino acid, in addition to their role as proton monomeric units, are energy
1) Essential amino acid
2) Non essential amino acid
While the non essential amino acid can be synchronized from metabolic processor, the essential amino acid must be provide by the diet. However, the essential dietary amino acid, instead of being extract or stored for future use, are converted to common metabolic intermediate like pyruvate, oxaloacetate, acetyl CoA, α-ketoglutarate etc. Thus amino acid are also precious of glucose, fatty acid, and
For the present, tyrosine clarifies itself to be a non essential amino acid, it is thus a metabolic processor and need not be supplied by the diet.
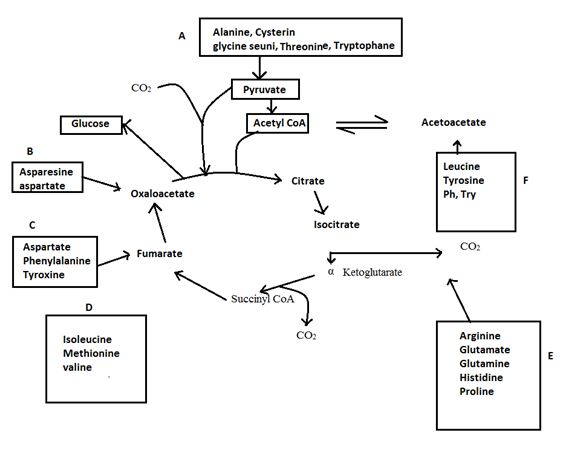
Further tyrisone is alos both a glucogenic and ketogenic amino acid. Its carbon skeleton can be degraded to either of pyruvate, α-ketoglutarate acetyl CoA, fumarate or oxaloacetate glucogenic amino acid and also to acetyl CoA or aceto acetate to be converted to ketone bodies or fatty acid. (ketogenic amino acid)
Degradation of amino acids to one of seven common metabolic intermediates
Want to see the full answer?
Check out a sample textbook solution
Chapter 29 Solutions
Organic Chemistry
- Differentiate between single links and multicenter links.arrow_forwardI need help on my practice final, if you could explain how to solve this that would be extremely helpful for my final thursday. Please dumb it down chemistry is not my strong suit. If you could offer strategies as well to make my life easier that would be beneficialarrow_forwardNonearrow_forward
- Definition and classification of boranes.arrow_forwardWhich of the terms explain the relationship between the two compounds? CH2OH Он Он Он Он α-D-galactose anomers enantiomers diastereomers epimers CH2OH ОН O он Он ОН B-D-galactosearrow_forwardHi, I need help on my practice final, If you could offer strategies and dumb it down for me with an explanation on how to solve that would be amazing and beneficial.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning


