
(a)
Interpretation: MOs for the given conjugated systems has to be drawn and the HOMO has to be predicted as symmetric or antisymmetric.
Concept introduction:
Conjugated system: A system of connected p-orbitals with delocalized electrons with alternating single and multiple bonds and the compound may be cyclic, linear or mixed.
Molecular orbital theory suggests that atomic orbitals of different atoms combines to create molecular orbitals.
Molecular orbitals can be constructed from linear combination of atomic orbitals.
Bonding orbotals are formed by the additive combination of atomic orbitals and the antibonding orbitals are formed by the substractive combination of atomic orbitals.
Antibonding orbital is a molecular orbital that results when two parallel atomic orbitals with opposite phases interact.
Antibonding orbitals have higher energy than the bonding molecular orbitals.
Ground state and and exited states are the positions with lower and higher energy respectively.
HOMO is a molecular orbital which is the abbrevation of Highest Occupied Molecular Orbital.
LUMO is also a molecular orbital which is the short form of Lowest Unoccupied Molecular Orbital.
If the lobes at the ends of the MO are in phase, then the MO is symmetric.
If the two lobes are out phase then the MO is antisymmetric.
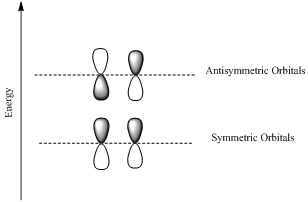
Woodward –Hoffmann rules are the set of rules used to vindicate or predict certain aspects of the stereo chemical outcome and activation energy of pericyclic reactions.
Woodward – Hoffmann rules for Electrocyclic reactions are listed below
(b)
Interpretation: The validity of Woodward – Hoffmann rule for an electrocyclic reaction has to be checked using the MOs of given systems.
Concept introduction:
Woodward –Hoffmann rules are the set of rules used to vindicate or predict certain aspects of the stereo chemical outcome and activation energy of pericyclic reactions.
Woodward – Hoffmann rules for Electrocyclic reactions are listed below
Want to see the full answer?
Check out a sample textbook solution
Chapter 28 Solutions
Organic Chemistry
- Refer Chapter 9 of "Physical Methods for Chemists" by RS DRAGO 1. How many hyperfine peaks would be expected from delocalization of the odd electron in dibenzene chromium cation onto the rings? 2.arrow_forward9. Would valence bond theory be expected to give a reliable description of the bonding in hex-2-enoic acid? Why or why not? 10. In 3 sentences or less, explain how the LCAO-MO technique enables molecular orbitals to be constructed.arrow_forwardPlease give clear handwritten answer of both subparts.arrow_forward
- In this experiment, we rely upon Infrared (IR) Spectroscopy to confirm that the expected reaction had occurred. Give the two uses of IR in organic chemistry. 1) Comparing known to unknown samples (peak by peak march) [fingerprint] 2) Determine the melting point of a solid compound. 3) Determine the presence or absence of functional groups or bonds in a molecule 4) Isolate particular functional groups for independent characterization 5) Determine the completion of a reaction by comparison of band intensitiesarrow_forward2. Kindly give what is asked in these problems.arrow_forwardConsider the reaction of two compounds ‘A’ and ‘B’ which could make two possible diastereomers ‘AB’ and ‘BA’ (much like this week’s Diels Alder reaction). Hand-write your calculations and responses to the following questions and upload your work as a .jpg or .pdf file. Which of the two products (A or B) will form in greater abundance under thermodynamic control? Which will form in greater abundance under kinetic control? Explain your responses using a sketch of the reaction coordinate diagram for the reactions described above.arrow_forward
- . Discuss the truth of the following statement. Explain why it is true or false Every SN1 reaction produces racemic mixtures in the productsarrow_forwardWhen free in solution (i.e., not bound to opsin), both all-trans and 11-cis retinal have absorption maxima of about 370-380 nm. a. How many electrons can be counted in the conjugated pi-system?b. Using l = 370 nm and the 1D particle in a box model, estimate the effective chain length of the retinal chromophore.arrow_forwardFind information on: 1. DDT 2. EDTA 3. orotic acid 4. сarboplatin 2. Propose the approximate shift/splitting pattern/etc. for each type of hydrogen that would be seen in an 'H-NMR spectrum Identify the # of each type of each carbon (i.e. how many peaks in the 13C spectra) 3. Identify infrared (IR) active bonds within each molecule 4.arrow_forward
- 6. Justify briefly based on bond energies and basic physics, the relative positions of IR absorptions corresponding to OH, alkene, alkyne, and carbonyl.arrow_forwardThoroughly describe what is meant when a compound is considered aromatic. In your answer, provide examples and be sure to discuss how Huckel's Rule applies, and what it means to be anti-aromatic versus non-aromatic. When providing specific examples to demonstrate the requirements that you list, describe the delocalized pi electrons and sp2 hybridization. Lastly, why is benzene so special compared to alkenes like cyclohexene....both are 6-carbon atom cycles so are they chemically similar or not really similar at all?arrow_forward8 to 1 (b) This carbon-carbon cross coupling is working effectively if R₂ is aryl group (Ph-X), but this catalytic reaction may fail if R₂ is alkyl group (e.g. n-Bu-X). Explain why this happens, and what you can do in order to ensure the success of the cross coupling of C(sp3)-C(sp2) (hint: consider environment of ligand and metal complex) Tu un utill for reaction. itarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
