
Organic Chemistry, Books a la Carte Edition (8th Edition)
8th Edition
ISBN: 9780134074580
Author: Bruice, Paula Yurkanis
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 27, Problem 44P
The
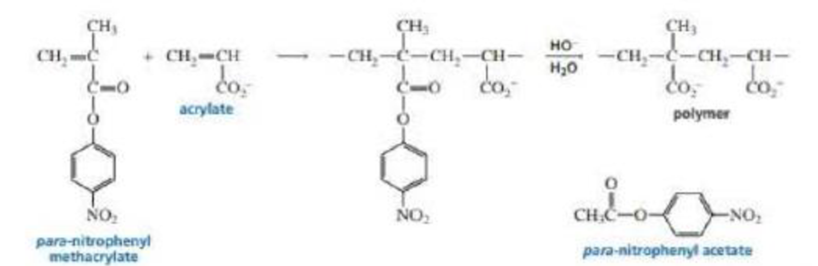
- a. Propose a mechanism for the formation of the copolymer.
- b. Explain why hydrolysis of the copolymer to form the polymer occurs much more rapidly than hydrolysis of para-nitrophenyl acetate.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
The polymer shown below is synthesized by hydroxide ion-promoted hydrolysis of a copolymer of para-nitrophenyl methacrylate and acrylate. a. Propose a mechanism for the formation of the copolymer. b. Explain why hydrolysis of the copolymer to form the polymer occurs much more rapidly than hydrolysis of para-nitrophenyl acetate.
1. which cannot be obtained by radical polymerization of its vinyl monomer
Poly(ethylene) obtained by polymerization of ethylene monomer
Poly(propylene) obtained by polymerization of propylene monomer
Poly(vinyl chloride) obtained by polymerization of vinyl chloride monomer
Poly(acrylonitrile) obtained by polymerization of acrylonitrile monomer
Which reaction—dehydration synthesis or hydrolysis—converts a polymer to its monomers? Which one convertsmonomers to a polymer? Explain your answer
Chapter 27 Solutions
Organic Chemistry, Books a la Carte Edition (8th Edition)
Ch. 27.3 - Prob. 1PCh. 27.3 - Prob. 2PCh. 27.3 - Prob. 3PCh. 27.3 - Prob. 4PCh. 27.3 - Prob. 5PCh. 27.3 - Prob. 6PCh. 27.4 - Prob. 7PCh. 27.5 - Rank the following groups of monomers from most...Ch. 27.5 - Why does methyl methacrylate not undergo cationic...Ch. 27.6 - Prob. 10P
Ch. 27.6 - Explain why, when propylene oxide undergoes...Ch. 27.6 - Which monomer and which type of initiator can you...Ch. 27.6 - Prob. 13PCh. 27.8 - Draw a short segment of gutta-percha.Ch. 27.8 - Prob. 15PCh. 27.11 - Prob. 16PCh. 27.11 - Write an equation that explains what happens if a...Ch. 27.11 - What happens to polyester slacks if aqueous NaOH...Ch. 27.11 - a. Propose a mechanism for the formation of the...Ch. 27.11 - Explain why, when a small amount of glycerol is...Ch. 27.12 - Propose a mechanism for the formation of melmac.Ch. 27.12 - Prob. 22PCh. 27.13 - Prob. 23PCh. 27 - Draw short segments of the polymers obtained from...Ch. 27 - Prob. 25PCh. 27 - Prob. 26PCh. 27 - Draw the structure of the monomer or monomers used...Ch. 27 - Prob. 28PCh. 27 - Draw short segments of the polymers obtained from...Ch. 27 - Quiana is a synthetic fabric that feels very much...Ch. 27 - Prob. 31PCh. 27 - Prob. 32PCh. 27 - Prob. 33PCh. 27 - Poly(vinyl alcohol) is a polymer used to make...Ch. 27 - Five different repeating units are found in the...Ch. 27 - Prob. 37PCh. 27 - A particularly strong and rigid polyester used for...Ch. 27 - Prob. 39PCh. 27 - Which Monomer gives a greater yield of polymer,...Ch. 27 - Prob. 41PCh. 27 - Prob. 42PCh. 27 - Why do vinyl raincoats become brittle as they get...Ch. 27 - The polymer shown below is synthesized by...Ch. 27 - Prob. 45PCh. 27 - How can head-to-head poly(vinyl bromide) be...Ch. 27 - Delrin (polyoxymethylene) is a tough...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Styrene derivatives such as A can be polymerized by way of cationic rather than radical intermediates. Cationic polymerization is an example of electrophilic addition to an alkene involving carbocations. a.) Draw a short segment of the polymer formed by the polymerization ofA.b.) Why does A react faster than styrene (C6H5CH=CH2) in a cationicpolymerization?arrow_forwardThe reaction of p-cresol with CH2=O resembles the reaction of phenol (PhOH) with CH2=O, except that the resulting polymer is thermoplastic but not thermosetting. Draw the structure of the polymer formed, and explain why the properties of these two polymers are so different.arrow_forwardWhich monomer gives a greater yield of polymer, 5-hydroxypentanoic acid or 6-hydroxyhexanoic acid? Explain your choice.arrow_forward
- Question 4. Methylvinyl ketone (MVK) can undergo a polymerization reaction in the presence of AIBN initiator to produce a poly(methylvinyl ketone) polymer according to the scheme below. NEC H3C CH3 `N=N₂ CH3 CEN CH3 azobisisobutyronitrile (AIBN) CH3 methylvinyl ketone (MVK) heat CH3 H3C NEC n poly(methylvinyl ketone) a) Provide the mechanism for heat-induced decomposition of the AIBN initiator to produce a reactive radical species. CH3 NEC H3C `N = N heat CH3 CEN CH3 10 0-0 b) Provide a mechanism for the initiation step in the MVK polymerization reaction: c) Show the mechanism for the propagation step to form an MVK oligomer with n = 3: branched sidare tass xy ha etarrow_forwardWhich statement about the reaction of benzene with Cl₂ in the presence of AICI3 is incorrect? a. The mechanism is electrophilic addition Chlorobenzene is formed b. Oc. The mechanism goes via an intermediate cation Od. AlCl3 is a catalystarrow_forwardThe reaction of p-cresol with CH2=O resembles the reaction of phenol (PHOH) with CH2 = 0, except that the resulting polymer is thermoplastic but not thermosetting. Draw the structure of the polymer formed, and explain why the properties of these two polymers are so different. CH3 OH + CH2=0 p-cresolarrow_forward
- How would you synthesize triphenylmethanol starting from the following class of compound? Draw the structure of the starting compound and list the other reagent(s) used for the reaction. a. Ester b. Ketone c. Aldehyde eduction dd H IT More "0" Oxidiz strong leak Reduarrow_forwardWhich of the following reactions will result in the formation of an acyl halide? Select one: a. The reaction of a carboxylic acid with phosphorus trichloride. b. The treatment of an alcohol with ethyl bromide. c. The reaction of an ester with hydrochloric acid. d. The addition of an alkene to dilute hydrochloric acid.arrow_forwardDraw a structural formula of the polymer resulting from base-catalyzed polymerization of each compound. Would you expect the polymers to be optically active? (S)-(+)-lactide is the dilactone formed from two molecules of (S)-(+)-lactic acid.arrow_forward
- Show the structure of the polymer that results from heating the following diepoxide and diamine:arrow_forwardWhat polymer is formed in each of the following reactions? Draw each polymer in polymer notation.n →> Catalyst Part B CH₂ Catalyst n CH H* 12D EXP. CONT. Marvin JS by ChemAxon A # H с N S CI Br - PFarrow_forwardWhat polymers are formed from each monomer? он о Он LOH OH b. H2N а.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:9781305446021
Author:Lampman
Publisher:CENGAGE LEARNING - CONSIGNMENT
CBSE Class 12 Chemistry || Polymers || Full Chapter || By Shiksha House; Author: Best for NEET;https://www.youtube.com/watch?v=OxdJlS0xZ0Y;License: Standard YouTube License, CC-BY