
Organic Chemistry
7th Edition
ISBN: 9780321803221
Author: Paula Y. Bruice
Publisher: Prentice Hall
expand_more
expand_more
format_list_bulleted
Question
Chapter 27, Problem 31P
Interpretation Introduction
Interpretation:
The reason for obtaining a random copolymer when
Concept Introduction:
Monomers combine together to form polymers. Monomers are the repeating units of small molecules which link together to form polymers and the process is called as polymerization.
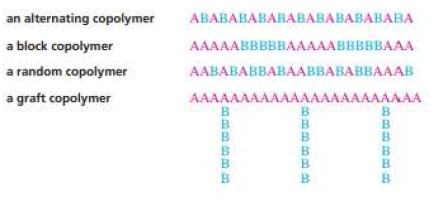
- Polymers formed from two or more different monomers are called copolymers.
- Classified into alternating copolymer, block copolymer, graft copolymer and also random copolymer.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Explain why a random copolymer is obtained when 3,3-dimethyl-1-butene undergoes cationic polymerization.
Explain why styrene (CH2=CHPh) can be polymerized to polystyrene by all three methods of chain-growth polymerization.
Draw the structure of the polymer that results from anionic polymerization of p-trichloromethylstyrene (CCl,CgH,CH=CH2) using
ethylene oxide as the electrophile to terminate the chain.
Chapter 27 Solutions
Organic Chemistry
Ch. 27.2 - Prob. 1PCh. 27.2 - Prob. 2PCh. 27.2 - Prob. 3PCh. 27.2 - Prob. 4PCh. 27.2 - Prob. 5PCh. 27.2 - Prob. 6PCh. 27.2 - Prob. 7PCh. 27.2 - Rank the following groups of monomers from most...Ch. 27.2 - Why does methyl methacrylate not undergo cationic...Ch. 27.2 - Explain why, when propylene oxide undergoes...
Ch. 27.2 - Prob. 11PCh. 27.2 - Which monomer and which type of initiator can you...Ch. 27.2 - Prob. 13PCh. 27.4 - Draw a short segment of gutta-percha.Ch. 27.4 - Prob. 15PCh. 27.7 - Prob. 16PCh. 27.7 - Write an equation that explains what happens if a...Ch. 27.7 - Prob. 18PCh. 27.7 - What happens to polyester slacks if aqueous NaOH...Ch. 27.7 - a. Propose a mechanism for the formation of the...Ch. 27.7 - Explain why, when a small amount of glycerol is...Ch. 27.8 - Propose a mechanism for the formation of melmac.Ch. 27.8 - Prob. 23PCh. 27.10 - Prob. 24PCh. 27 - Draw short segments of the polymers obtained from...Ch. 27 - Prob. 26PCh. 27 - Draw the structure of the monomer or monomers used...Ch. 27 - Prob. 28PCh. 27 - Draw short segments of the polymers obtained from...Ch. 27 - Quiana is a synthetic fabric that feels very much...Ch. 27 - Prob. 31PCh. 27 - Prob. 32PCh. 27 - Poly(vinyl alcohol) is a polymer used to make...Ch. 27 - Five different repeating units are found in the...Ch. 27 - Prob. 35PCh. 27 - A particularly strong and rigid polyester used for...Ch. 27 - Prob. 37PCh. 27 - Which Monomer gives a greater yield of polymer,...Ch. 27 - Prob. 39PCh. 27 - Prob. 40PCh. 27 - Why do vinyl raincoats become brittle as they get...Ch. 27 - The polymer shown below is synthesized by...Ch. 27 - Prob. 43PCh. 27 - How can head-to-head poly(vinyl bromide) be...Ch. 27 - Delrin (polyoxymethylene) is a tough...
Knowledge Booster
Similar questions
- Polymerization of vinyl acetate gives poly(vinyl acetate). Hydrolysis of this polymer in aqueous sodium hydroxide gives the useful water-soluble polymer poly(vinyl alcohol). Draw the repeat units of both poly(vinyl acetate) and poly(vinyl alcohol).arrow_forwardExplain why cationic polymerization is an effective method of polymerizing CH2 = C(CH3)2 but not CH2 = CH2.arrow_forwardCan you explain condensation polymerization and give an example with structure of one with 2 carboxylic acid groups and 2 OH groups.arrow_forward
- Draw the structure of the polymer formed from ring-opening metathesis polymerization (ROMP) of each monomer.arrow_forwardKodel is a condensation polymer made from terephthalic acid and 1,4-cyclohexanedimethanol. Write the structure of the resulting polymer.arrow_forwardExplain why acrylonitrile (CH2 = CHCN) undergoes cationic polymerization more slowly than but-3-enenitrile (CH2 = CHCH2CN).arrow_forward
- What polymer is formed when CH2=CHCO2H (acrylic acid) is polymerized toform poly(acrylic acid)?arrow_forwardDraw the curved arrow mechanism for the initiation step in cationic polymerization when methoxyethene reacts with BCI3 H₂O. Include unshared electrons and any nonzero formal charges. You do not need to show additional resonance forms. TA i Draw the curved arrow mechanism for the initiation step of this polymerization reaction. □ AN :CI: ‒‒‒‒‒‒‒ i 12D : CI: :CI—B :CI: + I H C N O S F P Cl Brarrow_forwardFill the following blanks 7/Number of isomers of the propane are --------. 8/Number of isomers of the Ethane are ------ 10/The melting of the parallel polymeric chains reduces --------. 11/Adding a plasticizer to the polymers reduces -------------arrow_forward
- Poly(ethylene terephthalate) (PET) can be prepared by this reaction. Propose a mecha- nism for the step-growth reaction in this polymerization. 275°C n CH;OC- -COCH, + n HOCH,CH,OH -COCH,CH,O- + 2n CH3OH Dimethyl terephthalate Ethylene glycol Poly(ethylene terephthalate) Methanolarrow_forward(a) Hard contact lenses, which first became popular in the 1960s, were made by polymerizing methyl methacrylate [CH2 = C(CH3)CO2CH3] to form poly(methyl methacrylate) (PMMA). Draw the structure of PMMA. (b) More comfortable softer contact lenses introduced in the 1970s were made by polymerizing hydroxyethyl methacrylate [CH2 = C(CH3)CO2CH2CH2OH] to form poly(hydroxyethyl methacrylate) (poly-HEMA). Draw the structure of poly-HEMA. Since neither polymer allows oxygen from the air to pass through to the retina, newer contact lenses that are both comfortable and oxygen-permeable have now been developed.arrow_forwardThe polymer forms NH-CH(CH3)–C, upon conden- sation polymerization with loss of water. Draw the struc- ture of the starting monomer.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning