
GENERAL CHEMISTRY-MOD.MASTERINGCHEM.
11th Edition
ISBN: 9780134193601
Author: Petrucci
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 26, Problem 10E
Interpretation Introduction
(a)
Interpretation:
The relationship between the following pair of molecules needs to be established:

Concept introduction:
- Isomers are two or more compounds which have the same formula but different structures and properties.
- Constitutional isomers are structural isomers which have the same molecular formula but different connectivity of the constituent atoms.
- Stereoisomers are spatial isomers which have the same formula and connectivity but differ in the orientation of the constituent atoms in space.
Interpretation Introduction
(b)
Interpretation:
The relationship between the following pair of molecules needs to be established:
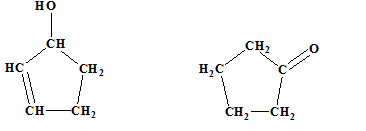
Concept introduction:
- Isomers are two or more compounds which have the same formula but different structures and properties.
- Constitutional isomers are structural isomers which have the same molecular formula but different connectivity of the constituent atoms.
- Stereoisomers are spatial isomers which have the same formula and connectivity but differ in the orientation of the constituent atoms in space.
Interpretation Introduction
(c)
Interpretation:
The relationship between the following pair of molecules needs to be established:
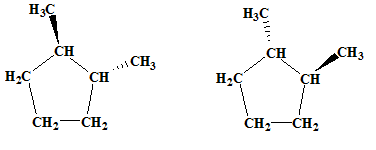
Concept introduction:
- Isomers are two or more compounds which have the same formula but different structures and properties.
- Constitutional isomers are structural isomers which have the same molecular formula but different connectivity of the constituent atoms.
- Stereoisomers are spatial isomers which have the same formula and connectivity but differ in the orientation of the constituent atoms in space.
Interpretation Introduction
(d)
Interpretation:
The relationship between the following pair of molecules needs to be established:
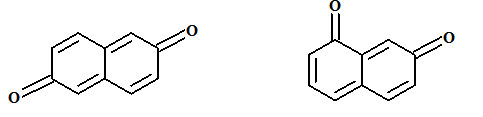
Concept introduction:
- Isomers are two or more compounds which have the same formula but different structures and properties.
- Constitutional isomers are structural isomers which have the same molecular formula but different connectivity of the constituent atoms.
- Stereoisomers are spatial isomers which have the same formula and connectivity but differ in the orientation of the constituent atoms in space.
Interpretation Introduction
(e)
Interpretation:
The relationship between the following pair of molecules needs to be established:
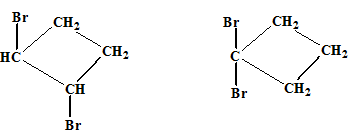
Concept introduction:
- Isomers are two or more compounds which have the same formula but different structures and properties.
- Constitutional isomers are structural isomers which have the same molecular formula but different connectivity of the constituent atoms.
- Stereoisomers are spatial isomers which have the same formula and connectivity but differ in the orientation of the constituent atoms in space.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
For each of the following pairs, give the relationship between the two compounds. You many ignore conformational isomerism here, and any conformers may be considered to be identical compounds.
Look at the following pairs of structures carefully to identify them as representing a) completely different compounds, b) compounds that are structural isomers of each other, c) compounds that are geometric isomers of each other, d) conformers of the same compound (part of structure rotated around a single bond) or e) the same structure.
Draw all possible isomers of trimethylcyclopropane. Name them. Which of these isomers are chiral? Indicate by underlining the name(s).
Chapter 26 Solutions
GENERAL CHEMISTRY-MOD.MASTERINGCHEM.
Ch. 26 - Prob. 1ECh. 26 - Draw a structural formula for each of the...Ch. 26 - Prob. 3ECh. 26 - Write structural formulas corresponding to these...Ch. 26 - Prob. 5ECh. 26 - Prob. 6ECh. 26 - Prob. 7ECh. 26 - Prob. 8ECh. 26 - What is the relationship, if any, between the...Ch. 26 - Prob. 10E
Ch. 26 - Prob. 11ECh. 26 - Prob. 12ECh. 26 - Identify the chiral carbon atoms, ¡f any, in the...Ch. 26 - Prob. 14ECh. 26 - Identify the chiral carbon atoms, ¡f any, in the...Ch. 26 - Prob. 16ECh. 26 - Prob. 17ECh. 26 - Prob. 18ECh. 26 - Prob. 19ECh. 26 - By name or formula, give one example of each of...Ch. 26 - Prob. 21ECh. 26 - Prob. 22ECh. 26 - Prob. 23ECh. 26 - Prob. 24ECh. 26 - Prob. 25ECh. 26 - Prob. 26ECh. 26 - Prob. 27ECh. 26 - Prob. 28ECh. 26 - Prob. 29ECh. 26 - Prob. 30ECh. 26 - Prob. 31ECh. 26 - Prob. 32ECh. 26 - Prob. 33ECh. 26 - Prob. 34ECh. 26 - Does each of the following names convey sufficient...Ch. 26 - Prob. 36ECh. 26 - Prob. 37ECh. 26 - Supply condensed structural formulas for the...Ch. 26 - Prob. 39ECh. 26 - Prob. 40ECh. 26 - Classify the carbon atoms in, a. methylbutane, and...Ch. 26 - Classity the carbon atoms in a....Ch. 26 - Prob. 43ECh. 26 - Draw Newman projections for the staggered and...Ch. 26 - Draw the most stable conformation for the molecule...Ch. 26 - Prob. 46ECh. 26 - Prob. 47ECh. 26 - Prob. 48ECh. 26 - Prob. 49ECh. 26 - Prob. 50ECh. 26 - Prob. 51ECh. 26 - Prob. 52ECh. 26 - Prob. 53ECh. 26 - Prob. 54ECh. 26 - Prob. 55ECh. 26 - Prob. 56ECh. 26 - Draw suitable structural formulas to show that...Ch. 26 - Which of the following pairs of molecules are...Ch. 26 - Prob. 59ECh. 26 - Prob. 60ECh. 26 - Name the following molecules with the appropriate...Ch. 26 - Name the following molecules with the appropriate...Ch. 26 - Name the following molecules with the appropriate...Ch. 26 - Prob. 64ECh. 26 - Draw the structure for each of the following. a....Ch. 26 - Prob. 66ECh. 26 - Prob. 67ECh. 26 - Prob. 68ECh. 26 - Prob. 69ECh. 26 - Prob. 70ECh. 26 - Prob. 71ECh. 26 - Prob. 72ECh. 26 - Prob. 73ECh. 26 - Prob. 74ECh. 26 - Supply condensed or structural formulas for the...Ch. 26 - Prob. 76IAECh. 26 - Prob. 77IAECh. 26 - Prob. 78IAECh. 26 - Prob. 79IAECh. 26 - Prob. 80IAECh. 26 - Combustion of a 0.1908 g sample of a compound gave...Ch. 26 - Prob. 82IAECh. 26 - In the monochiorination of hydrocarbons, a...Ch. 26 - A particular colorless organic liquid is known to...Ch. 26 - Prob. 85IAECh. 26 - Give the systematic names, including any...Ch. 26 - Prob. 87IAECh. 26 - Prob. 88IAECh. 26 - Levomethadyl acetate (shown below) is used in the...Ch. 26 - Thiamphenicol (shown below) is an antibacterial...Ch. 26 - Prob. 91IAECh. 26 - Prob. 92IAECh. 26 - Prob. 93IAECh. 26 - Prob. 94IAECh. 26 - Prob. 95IAECh. 26 - For each of the following molecules (a) draw the...Ch. 26 - Prob. 97FPCh. 26 - Prob. 98SAECh. 26 - Explain the important distinctions between each...Ch. 26 - Describe the characteristics of each of the...Ch. 26 - The compound isoheptane is best represented by the...Ch. 26 - Prob. 102SAECh. 26 - Prob. 103SAECh. 26 - Prob. 104SAECh. 26 - Assign configurations, R or S, to the chiral...Ch. 26 - Consider the following pairs of structures In each...Ch. 26 - Prob. 107SAECh. 26 - Prob. 108SAECh. 26 - Prob. 109SAECh. 26 - Prob. 110SAECh. 26 - Prob. 111SAE
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Why are different conformations of an alkane not considered structural isomers?arrow_forward2-methylhexane & 3,3-dimethylpentane Are the two compounds Constitutional Isomers or Different Compounds? Draw structures for both compounds.arrow_forwardS.3. Show in each of the pairs below whether the two structures are structural isomers, stereoisomers, or different Conformations of the same compound.arrow_forward
- Octane has how many isomers? How do I determine? Noarrow_forwardConsider the following representation of 1,2-dimethylcyclopentane.Which of the following describes the molecule represented? cis or trans isomer or noneWhat is the molecular formula (in the order CH) of the compound?arrow_forwardHow many structural isomers of the molecular formula C₃H₆O do not have cyclic structures? Hint: You need to consider placing more than one functional group in a molecule.arrow_forward
- Label following pairs of molecules as being either same same structure, completely different, constitutional isomers, or stereoisomers.arrow_forwardIt is easy to imagine a cyclohexane as a flat hexagon and a lot of the time we draw it that way. Looking at 1,3,5-triethylcyclohexane we cannot tell the stability of the molecule from looking at the flat 2D drawing. Explain why we need to look at the 3D configuration and what conformation (axial,equatorial) would each of the three ethyl groups be in for the most stable configuration.arrow_forward3. How many kinds of H are there in the isomers of dimethylcyclopropane?arrow_forward
- Explain What are conformational isomerism?arrow_forwardWhat is the relationship for the following pairs of compounds. The options include constitutional isomers, not isomers, enantiomers, conformers, identical, resonance structures.arrow_forwardWhich of the following statements are correct? * Conformation exist in a dynamic equilibrium state. Conformers differ largely in energies. Conformers cannot be separated. Amount of more stable conformers is more than that of less stable conformers at equilibrium.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License