
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
What is the relationship for the following pairs of compounds. The options include constitutional isomers, not isomers, enantiomers, conformers, identical, resonance structures.
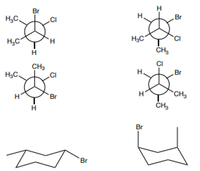
Transcribed Image Text:H.
Br
Br
H,C.
H3C
ČH,
'CI
H,C
CI
CH3
Br
H3C.
CH3
Br
Br
Br
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Do a conformational analysis for the following molecules (include energy diagram). H₂C- Hillm Br → H will H H F Br ull Brarrow_forwardWe have learned that isomers (molecules with the same molecular formula) can have different relationships, and these relationships can be made more clear by comparing IUPAC names. Constitutional isomers will differ in the main part of the name. Stereoisomers will differ only in the stereochemical prefix part of the name (cis/trans). Conformational isomers will actually have the same name, but different energies. Two structures that have the same names and the same energies are identical. What is the relationship between these two structures?arrow_forwardOn the following energy diagram, identify the energy level of the cyclohexane conformation shown in the box. Energy (kJ/mol) A) I B) II CLU ||| IV Conformations 2arrow_forward
- For which isomer would you expect a greater equilibrium percentage of molecules with the alkyl group in the axial position, isopropylcyclohexane or propylcyclohexane? Explain. Isopropylcyclohexane Propylcyclohexanearrow_forwardWe have learned that isomers (molecules with the same molecular formula) can have different relationships, and these relationships can be made more clear by comparing IUPAC names. Constitutional isomers will differ in the main part of the name. Stereoisomers will differ only in the stereochemical prefix part of the name (cis/trans). Conformational isomers will actually have the same name, but different energies. Two structures that have the same names and the same energies are identical. What is the relationship between these two structures?arrow_forward(a) All Isomers have the same molecular formulae. Explain this further. Hint what does it mean to have the same molecular formulae? (b) What is Constitutional (structural) isomerism? Hint, isomers are different, so what is different between a pair of constitutional isomers (see the chart on the last page for examples). (c) What is Conformational isomerism?arrow_forward
- How do you account for the difference in energies between the two staggered conformations of 1,2-dichloroethane? How about for the two eclipsed conformations? Draw all four conformations and, on your drawing, indicate sources of strain – torsional, steric (gauche), steric eclipsed.arrow_forwardChemistry Determine how many substituents in the axial position does the most stable conformer of (1R,2S,4R)-4-bromo-1,2-dimethylcyclohexane have?arrow_forward(a) What is the IUPAC name for the following molecule (including absolute configurations if required)? (b) Draw the Newman projections looking down the C3-C4 bond that represent all the three staggered conformations for the molecule shown above. (c) Given that the strain energies below, calculate the strain energies for each conformer and identify which is the most stable. Gauche interaction Strain Energy HOCH3 0.0 kJ mol- HOCH;CH; |0.4 kJ mol- 3.8 kJ mol- | 4.3 kJ mol- CH;CH3 CH;CH2CH3 |CH2CH; CH2CH; 4.6 kJ mol- Newman Projection of Conformer 1 Strain Energy of Conformer 1 Newman Projection of Conformer 2 Strain Energy of Conformer 2 Newman Projection of Conformer 3 Strain Energy of Conformer 3arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY