
Concept explainers
(a)
Interpretation:
The product formed by the reaction of p-methylaniline
Concept introduction:
Synthesis is one of the major areas in the field of
The inorganic reagents do not contain a carbon atom. These reagents generally contain inorganic molecules, but sometimes small organic molecule also acts as an inorganic reagent. The reagents are additional chemical substances that are used to perform various
Answer to Problem 25.61P
The product formed by the reaction of p-methylaniline
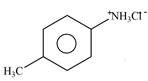
Explanation of Solution
The given reagent is
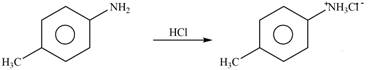
Figure 1
The nitrogen atom of p-methylaniline is basic in nature. It abstracts proton from
The product formed by the reaction of p-methylaniline
(b)
Interpretation:
The product formed by the reaction of p-methylaniline
Concept introduction:
Synthesis is one of the major areas in the field of organic chemistry. It can be a simple one-step reaction or it may involve many steps.
The inorganic reagents do not contain a carbon atom. These reagents generally contain inorganic molecules, but sometimes small organic molecule also acts as an inorganic reagent. The reagents are additional chemical substances that are used to perform various chemical reactions like reduction, oxidation, base, catalysis, and the addition of functional groups.
Answer to Problem 25.61P
The products formed by the reaction of p-methylaniline
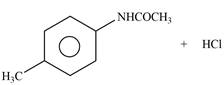
Explanation of Solution
The given reagent is
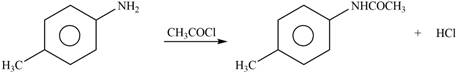
Figure 2
In the above reaction, acetyl chloride dissociates to form
The products formed by the reaction of p-methylaniline
(c)
Interpretation:
The product formed by the reaction of p-methylaniline
Concept introduction:
Synthesis is one of the major areas in the field of organic chemistry. It can be a simple one-step reaction or it may involve many steps.
The inorganic reagents do not contain a carbon atom. These reagents generally contain inorganic molecules, but sometimes small organic molecule also acts as an inorganic reagent. The reagents are additional chemical substances that are used to perform various chemical reactions like reduction, oxidation, base, catalysis, and the addition of functional groups.
Answer to Problem 25.61P
The products formed by the reaction of p-methylaniline
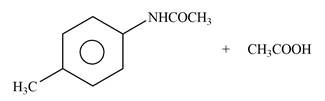
Explanation of Solution
The given reagent is
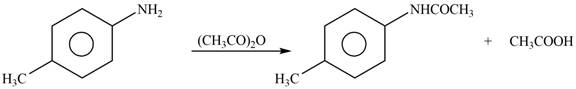
Figure 3
In the above reaction, the nucleophilic nitrogen atom attacks at the electrophilic carbonyl carbon atom of acetic anhydride to form the final product amide.
The products formed by the reaction of p-methylaniline
(d)
Interpretation:
The product formed by the reaction of p-methylaniline
Concept introduction:
Synthesis is one of the major areas in the field of organic chemistry. It can be a simple one-step reaction or it may involve many steps.
The inorganic reagents do not contain a carbon atom. These reagents generally contain inorganic molecules, but sometimes small organic molecule also acts as an inorganic reagent. The reagents are additional chemical substances that are used to perform various chemical reactions like reduction, oxidation, base, catalysis, and the addition of functional groups.
Answer to Problem 25.61P
The product formed by the reaction of p-methylaniline
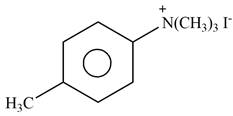
Explanation of Solution
The given reagent is excess
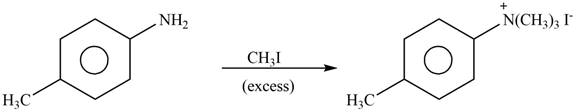
Figure 4
In the above reaction, p-methylaniline recats with excess of methyl iodide to form
The product formed by the reaction of p-methylaniline
(e)
Interpretation:
The product formed by the reaction of p-methylaniline
Concept introduction:
Synthesis is one of the major areas in the field of organic chemistry. It can be a simple one-step reaction or it may involve many steps.
The inorganic reagents do not contain a carbon atom. These reagents generally contain inorganic molecules, but sometimes small organic molecule also acts as an inorganic reagent. The reagents are additional chemical substances that are used to perform various chemical reactions like reduction, oxidation, base, catalysis, and the addition of functional groups.
Answer to Problem 25.61P
The products formed by the reaction of p-methylaniline
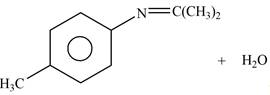
Explanation of Solution
The given reagent is
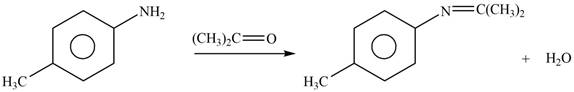
Figure 5
In the above reaction, p-methylaniline reacts with acetone to form the final product.
The products formed by the reaction of p-methylaniline
(f)
Interpretation:
The product formed by the reaction of p-methylaniline
Concept introduction:
Synthesis is one of the major areas in the field of organic chemistry. It can be a simple one-step reaction or it may involve many steps.
The inorganic reagents do not contain a carbon atom. These reagents generally contain inorganic molecules, but sometimes small organic molecule also acts as an inorganic reagent. The reagents are additional chemical substances that are used to perform various chemical reactions like reduction, oxidation, base, catalysis, and the addition of functional groups.
Answer to Problem 25.61P
The product formed by the reaction of p-methylaniline
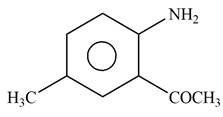
Explanation of Solution
The given reagent is
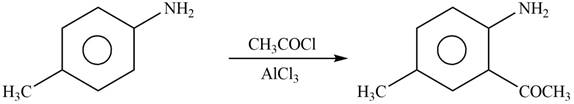
Figure 6
In the above reaction, acetyl chloride dissociates to form
The product formed by the reaction of p-methylaniline
(g)
Interpretation:
The product formed by the reaction of p-methylaniline
Concept introduction:
Synthesis is one of the major areas in the field of organic chemistry. It can be a simple one-step reaction or it may involve many steps.
The inorganic reagents do not contain a carbon atom. These reagents generally contain inorganic molecules, but sometimes small organic molecule also acts as an inorganic reagent. The reagents are additional chemical substances that are used to perform various chemical reactions like reduction, oxidation, base, catalysis, and the addition of functional groups.
Answer to Problem 25.61P
The product formed by the reaction of p-methylaniline
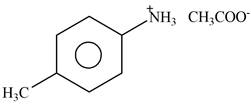
Explanation of Solution
The given reagent is

Figure 7
The nitrogen atom of p-methylaniline is basic in nature. It abstracts proton from
The product formed by the reaction of p-methylaniline
(h)
Interpretation:
The product formed by the reaction of p-methylaniline
Concept introduction:
Synthesis is one of the major areas in the field of organic chemistry. It can be a simple one-step reaction or it may involve many steps.
The inorganic reagents do not contain a carbon atom. These reagents generally contain inorganic molecules, but sometimes small organic molecule also acts as an inorganic reagent. The reagents are additional chemical substances that are used to perform various chemical reactions like reduction, oxidation, base, catalysis, and the addition of functional groups.
Answer to Problem 25.61P
The product formed by the reaction of p-methylaniline
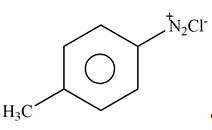
Explanation of Solution
The given reagent is
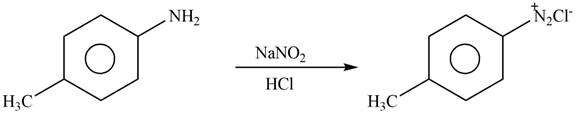
Figure 8
Diazonium salts are prepared by the treatment of
: The product formed by the reaction of p-methylaniline
(i)
Interpretation:
The product formed by the reaction of p-methylaniline
Concept introduction:
Synthesis is one of the major areas in the field of organic chemistry. It can be a simple one-step reaction or it may involve many steps.
The inorganic reagents do not contain a carbon atom. These reagents generally contain inorganic molecules, but sometimes small organic molecule also acts as an inorganic reagent. The reagents are additional chemical substances that are used to perform various chemical reactions like reduction, oxidation, base, catalysis, and the addition of functional groups.
Answer to Problem 25.61P
The product formed by the reaction of p-methylaniline
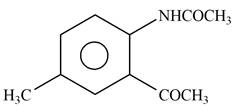
Explanation of Solution
The given reagent is
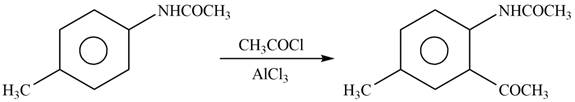
Figure 9
In the above reaction, acetyl chloride dissociates to form
The product formed by the reaction of p-methylaniline
(j)
Interpretation:
The product formed by the reaction of P-methylaniline
Concept introduction:
Synthesis is one of the major areas in the field of organic chemistry. It can be a simple one-step reaction or it may involve many steps.
The inorganic reagents do not contain a carbon atom. These reagents generally contain inorganic molecules, but sometimes small organic molecule also acts as an inorganic reagent. The reagents are additional chemical substances that are used to perform various chemical reactions like reduction, oxidation, base, catalysis, and the addition of functional groups.
Answer to Problem 25.61P
The product formed by the reaction of p-methylaniline
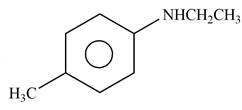
Explanation of Solution
The given reagent is
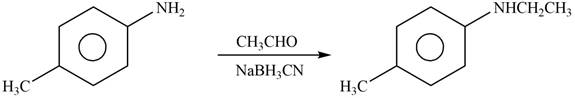
Figure 10
When an
The product formed by the reaction of p-methylaniline
Want to see more full solutions like this?
Chapter 25 Solutions
Organic Chemistry
- 5. What is the product? A. HCI H₂C CH₂ HOCH D. A and B A. A B. B C. C D. D E. E -CH3 2NaNH₂ H₂C B. C=CH H CH3 E. A, B and C C. H₂C-CEC-CH3arrow_forward16. Identify each compound as an a cohol, a phenol, or an ether. Classify any alcohols as primary (1"), secondary (2), or tertiary (3"). a. CH,CH,CH,OH CH,CHCH, b. CHO C. CH CHOCH, CH, d.arrow_forward22. How many of these reactions produce a product named 2-butanol? H₂SO4 H₂O A Br₂ CH₂Cl₂ A. 1 B. 2 C. 3 D. 4 E. 5 1.03 2. Zn/H₂O 1. BH 2. HyOz, NaOH 1. Hg(OAc)2, H₂O 2. NaBH4arrow_forward
- 32. Which of the following reactions can be used to prepare 3-methyl-3-hexanol, OH I CH3CH2CCH2CH2CH3 ? 1 CH 3 A. B. CH3CH2CCH2CH2CH3 i CH3CCH2CH2CH3 i CH3CCH2CH3 1. CH3MgBr 2. H30 C. D. A and B E. A, B, and C 1. CH3CH2MgBr 2. H30 1. CH3CH2CH2MgBr 2. H30 33. Complete the following reaction sequence by supplying the missing information: (1) (2) 4. ? CH3OH (3) CH3Br CH3CH2OH 5. ? A. (1) NaBr; (2) Mg/ether; (3) CH3MgBr; (4) H2C=0; (5) H2O B. (1) HBr; (2) Mg/ether; (3) CH3MgBr; (4) H2C=0; (5) H2O c. (1) Br2; (2) Mg/ether; (3) CH3MgBr; (4) H2C=O; (5) H2O D. (1) HBr; (2) CH3MgBr; (3) Mg/ether; (4) H2C=O; (5) H2Oarrow_forwardDraw the products formed when CH3CH2C=C Na+ reacts with each compound. a. CH3CH2CH2Br b.(CH3)2CHCH2CH2Cl c.(CH3CH2)3CCl d.BrCH2CH2CH2CH2OH e. ethylene oxide followed by H2O f.propene oxide followed by H2Oarrow_forwardReaction Product/s a. C,H1, + O2 b. Butane+ CI, c. Propanearrow_forward
- Draw the structure of a dihalide that could be used to prepare each alkyne. There may be more than one possible dihalide. CH3 b. CH₂-C-C=CH CH₂ a. CH₂C=CCH₂ C. -C=C-arrow_forward7. Name the following phenols. b) OH c) d) a) OH Br- HO CI Br 8. Name the following ethers. b) a) CHy-0-CH-CHy CH-0-CH3 c) d) Cゅ-C-oarrow_forwardWhat product(s) will form: 2-Pentene + H₂O → O a. 2-Pentanol O b. Isopropyl alcohol O c. 3-Pentanol O d. a 50/50 mixture of 2-Pentanol and 3-Pentanol Oe. no reaction will occurarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





