
Organic Chemistry (6th Edition)
6th Edition
ISBN: 9781260119107
Author: Janice Gorzynski Smith
Publisher: McGraw Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 24, Problem 39P
When certain cycloalkenes are used in metathesis reactions, ring-opening
metathesis
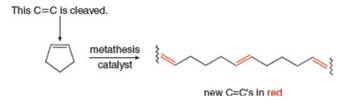
What products are formed by ring-opening metathesis polymerization of each
a.  b.
b. 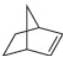 c.
c. 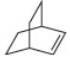
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Styrene derivatives such as A can be polymerized by way of cationic rather than radical intermediates. Cationic polymerization is an example of electrophilic addition to an alkene involving carbocations.
a.) Draw a short segment of the polymer formed by the polymerization ofA.b.) Why does A react faster than styrene (C6H5CH=CH2) in a cationicpolymerization?
Which compound is second least stable, giving off
the second most heat of hydrogenation or
combustion (when burned)?
a.
O b.
d.
Of.
As we will learn in Chapter 28, styrene derivatives such as A can be polymerized by way of cationic rather than radical intermediates. Cationic polymerization is an example of electrophilic addition to an alkene involving carbocations.a.Draw a short segment of the polymer formed by the polymerization of A. b.Why does A react faster than styrene (C6H5CH=CH2) in a cationic polymerization?
Chapter 24 Solutions
Organic Chemistry (6th Edition)
Ch. 24.1 - Prob. 1PCh. 24.1 - Prob. 2PCh. 24.1 - Prob. 3PCh. 24.2 - Prob. 4PCh. 24.2 - Prob. 5PCh. 24.4 - Problem 26.10
What reagents are needed to convert...Ch. 24.5 - Problem 26.11
What product is formed when each...Ch. 24.5 - Prob. 13PCh. 24.6 - Problem 26.13
Draw the products formed when each...Ch. 24.6 - Problem 26.14
What products are formed when ...
Ch. 24 - 26.19 What product is formed by ring-closing...Ch. 24 - 26.20 Draw the products formed in each...Ch. 24 - What organic halide is needed to convert lithium...Ch. 24 - 26.22 How can you convert ethynylcyclohexane to...Ch. 24 - 26.23 What compound is needed to convert styrene...Ch. 24 - 26.24 What steps are needed to convert to octane...Ch. 24 - Prob. 27PCh. 24 - 26.27 Draw the products (including stereoisomers)...Ch. 24 - 26.28 Treatment of cyclohexene with and forms...Ch. 24 - Prob. 32PCh. 24 - 26.30 What starting material is needed to prepare...Ch. 24 - Prob. 37PCh. 24 - Prob. 38PCh. 24 - When certain cycloalkenes are used in metathesis...Ch. 24 - 26.34 Draw the products formed in each reaction.
...Ch. 24 - Prob. 41PCh. 24 - Draw a stepwise mechanism for the following...Ch. 24 - Sulfur ylides, like the phosphorus ylides of...Ch. 24 - Although diazomethane is often not a useful...Ch. 24 - Prob. 46PCh. 24 - Prob. 47PCh. 24 - 26.45 Devise a synthesis of each compound from...Ch. 24 - 26.46 Devise a synthesis of each substituted...Ch. 24 - Biaryls, compounds containing two aromatic rings...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- An organic compound A reacts with sodium metal and forms B. On heating with conc H2SO4, A gives diethyl ether. What are A and B?arrow_forward4.2) Draw the structure of the polymer produced in the following reaction. H₂N NH₂arrow_forwardIsotretinoin is a retinoid derivative of vitamin A used in the treatment of severe recalcitrant acne. What is the degree of substitution of the encircled alkene functional group in its structure? ... А tetrasubstituted B) trisubstituted c) monosubstituted D disubstitutedarrow_forward
- Synthesize attached compound from toluene (C6H5CH3) and any other organic or inorganic reagents.arrow_forwardOnce reacted with NaNH2 in NH3, which forms the smallest number of methylated anilines? А. C. D. В, C .CI B.arrow_forward10.38 Give the IUPAC name for each compound. а. b. С. d. ОН е. f.arrow_forward
- Naftifine is a synthetic antifungal agent for topical treatment of tinea pedis, tinea cruris, and tinea corporis caused by organism rubrum. Trichophyton mentagrophytes, Trichophyton tonsurans, and Epidermophyton floccosum. What is the degree of substitution of Naftitine? (alkene found in the middle of the structure) a. trisubstituted b. monosubstituted c. disubstituted d. tetrasubstitutedarrow_forward4.2) Draw the structure of the polymer produced in the following reaction. H₂N NH₂ Karrow_forwardMCQ 36: Alkenes mostly follow the reaction of A. addition reaction B. elimination reaction C. substitution reaction D. none of abovearrow_forward
- Nomenclature 20.36 Give the IUPAC or common name for each compound. معلم a. b. C. H .N e. བྱིན ཏེ། LOCH 3 Br CIarrow_forward18. How many isomers does dichlorocyclobutane have? Consider both constitutional isomers and stereoisomers! A.2 B. 3 C. 4 D. 5 E. 6arrow_forwardWhen certain cycloalkenes are used in metathesis reactions, ringopening metathesis polymerization (ROMP) occurs to form a highmolecular- weight polymer, as shown with cyclopentene as the starting material. The reaction is driven to completion by relief of strain in the cycloalkene.What products are formed by ring-opening metathesis polymerization of each alkene?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
CBSE Class 12 Chemistry || Polymers || Full Chapter || By Shiksha House; Author: Best for NEET;https://www.youtube.com/watch?v=OxdJlS0xZ0Y;License: Standard YouTube License, CC-BY