
Concept explainers
(a)
Interpretation: To characterize Step 1 relative to the type of reaction that occurs in the glycolysis process.
Concept introduction: Glycolysis is the
The block diagram to represent an overview of glycolysis is as follows:
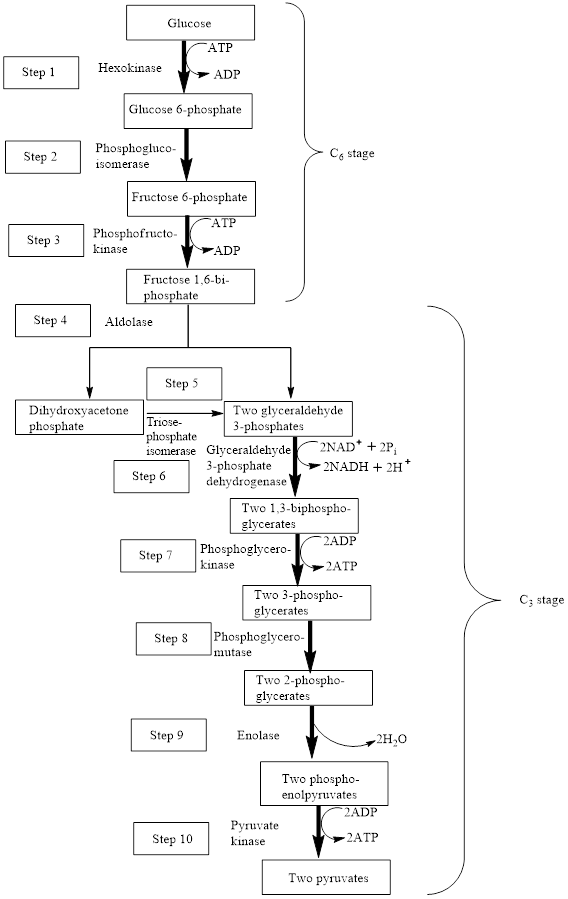
From the above diagram, it is concluded that in the overall process of glycolysis, two stages are present.
a) Steps 1 to 3 represents a six-carbon stage
b) Steps 4 to 10 represent a three-carbon stage
In the phosphorylation reaction, the molecule is attached to the phosphoryl group. The transfer of a phosphoryl group
In the isomerization reaction, a molecule transformed itself to another molecule, having the same number of atoms with a different arrangement.
(b)
Interpretation: To characterize step 3 relative to the type of reaction that occurs in the glycolysis process.
Concept introduction: Glycolysis is the metabolic pathway that breaks down a glucose molecule and converts it into two pyruvate molecules along with the production of two ATP molecules and NADH coenzymes.
The block diagram to represent an overview of glycolysis is as follows:

From the above diagram, it is concluded that in the overall process of glycolysis, two stages are present.
a) Steps 1 to 3 represents a six-carbon stage
b) Steps 4 to 10 represent a three-carbon stage
In the phosphorylation reaction, the molecule is attached to the phosphoryl group. The transfer of a phosphoryl group
In the isomerization reaction, a molecule transformed itself to another molecule, having the same number of atoms with a different arrangement.
(c)
Interpretation: To characterize step 5 relative to the type of reaction that occurs in the glycolysis process.
Concept introduction: Glycolysis is the metabolic pathway that breaks down a glucose molecule and converts it into two pyruvate molecules along with the production of two ATP molecules and NADH coenzymes.
The block diagram to represent an overview of glycolysis is as follows:

From the above diagram, it is concluded that in the overall process of glycolysis, two stages are present.
a) Steps 1 to 3 represents a six-carbon stage
b) Steps 4 to 10 represent a three-carbon stage
In the phosphorylation reaction, the molecule is attached to the phosphoryl group. The transfer of a phosphoryl group
In the isomerization reaction, a molecule transformed itself to another molecule, having the same number of atoms with a different arrangement.
(d)
Interpretation: To characterize step 5 relative to the type of reaction that occurs in the glycolysis process.
Concept introduction: Glycolysis is the metabolic pathway that breaks down a glucose molecule and converts it into two pyruvate molecules along with the production of two ATP molecules and NADH coenzymes. The conversion of a glucose molecule to the pyruvate molecules is an oxidation process in which no molecular
The block diagram to represent an overview of glycolysis is as follows:

From the above diagram, it is concluded that in the overall process of glycolysis, two stages are present.
a) Steps 1 to 3 represents a six-carbon stage
b) Steps 4 to 10 represent a three-carbon stage
In the phosphorylation reaction, the molecule is attached to the phosphoryl group. The transfer of a phosphoryl group
In the isomerization reaction, a molecule transformed itself to another molecule, having the same number of atoms with a different arrangement.
Want to see the full answer?
Check out a sample textbook solution
Chapter 24 Solutions
Study Guide with Selected Solutions for Stoker's General, Organic, and Biological Chemistry, 7th
- Ph heat heatarrow_forward(12) Which one of the following statements about fluo- rometry is FALSE? a) Fluorescence is better detected at 90 from the exci- tation direction. b) Fluorescence is typically shifted to longer wave- length from the excitation wavelength. c) For most fluorescent compounds, radiation is pro- duced by a transitionarrow_forwardDon't used Ai solutionarrow_forward
- (f) SO: Best Lewis Structure 3 e group geometry:_ shape/molecular geometry:, (g) CF2CF2 Best Lewis Structure polarity: e group arrangement:_ shape/molecular geometry: (h) (NH4)2SO4 Best Lewis Structure polarity: e group arrangement: shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles): Sketch (with angles):arrow_forward1. Problem Set 3b Chem 141 For each of the following compounds draw the BEST Lewis Structure then sketch the molecule (showing bond angles). Identify (i) electron group geometry (ii) shape around EACH central atom (iii) whether the molecule is polar or non-polar (iv) (a) SeF4 Best Lewis Structure e group arrangement:_ shape/molecular geometry: polarity: (b) AsOBr3 Best Lewis Structure e group arrangement:_ shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles):arrow_forward(c) SOCI Best Lewis Structure 2 e group arrangement: shape/molecular geometry:_ (d) PCls Best Lewis Structure polarity: e group geometry:_ shape/molecular geometry:_ (e) Ba(BrO2): Best Lewis Structure polarity: e group arrangement: shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles): Sketch (with angles):arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry In FocusChemistryISBN:9781305084476Author:Tro, Nivaldo J., Neu, Don.Publisher:Cengage Learning
Chemistry In FocusChemistryISBN:9781305084476Author:Tro, Nivaldo J., Neu, Don.Publisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





