
Sulfide ion (S2- ) is formed in wastewater by the action of an aerobic bacteria on organic matter. Sulfide can be readily protonated to form volatile, toxic H2S. In addition to the toxicity and noxious odor, sulfide and H2S cause corrosion problems because they can be easily converted to sulfuric acid when conditions change to aerobic. One common method to determine sulfide is by coulometric titration with generated silver ion.At the generator electrode, the reaction is Ag
(a) A digital chloridometer was used to determine the mass of sulfide in a wastewater sample. The chloridometer reads out directly in ng Cl-.In chloride determinations, the same generator reaction is used,but the titration reaction is Cl- + Ag+
(b) A particular wastewater standard gave a reading of 1689.6 ng Cl-. What total charge in coulombs was required to generate the Ag+ needed to precipitate the sulfide in this standard?
(c) The following results were obtained on 20.00-mL samples containing known amounts of sulfide.17
Each standard was analyzed in triplicate and the mass of chloride recorded. Convert each of the chloride results to mass S2- (ng).
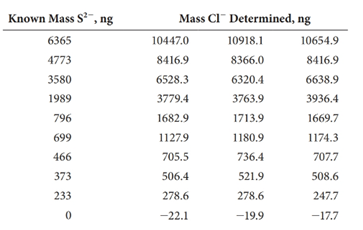
(d) Determine the average mass of S2- (ng), the standard deviation, and the %RSD) of each standard.
(e) Prepare a plot ofthe average mass of S2- determined (ng) versus the actual mass (ng). Determine theslope, the intercept, the standard error, and the R2 value. Comment on the fit of the data to a linear model.
(f) Determine the detection limit (ng) and in parts per million using a k factor of 2 (see Equation 1-12).
(g) An unknown wastewater sample gave an average reading of 893.2 ng Cl. What is the mass of sulfide (ng)? If 20.00 mL of the wastewater sample was introduced into the titration vessel, what is the concentration of S2- n parts per million?
Want to see the full answer?
Check out a sample textbook solution
Chapter 24 Solutions
Principles of Instrumental Analysis
- Definition and classification of boranes.arrow_forwardWhich of the terms explain the relationship between the two compounds? CH2OH Он Он Он Он α-D-galactose anomers enantiomers diastereomers epimers CH2OH ОН O он Он ОН B-D-galactosearrow_forwardHi, I need help on my practice final, If you could offer strategies and dumb it down for me with an explanation on how to solve that would be amazing and beneficial.arrow_forward
- Hi I need help with my practice final, it would be really helpful to offer strategies on how to solve it, dumb it down, and a detailed explanation on how to approach future similar problems like this. The devil is in the details and this would be extremely helpfularrow_forwardIn alpha-NbI4, Nb4+ should have the d1 configuration (bond with paired electrons: paramagnetic). Please comment.arrow_forwardHi, I need help on my practice final, if you could explain how to solve it offer strategies and dumb it down that would be amazing. Detail helpsarrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning





