
Concept explainers
(a)
Interpretation:
The structure of the product has to be drawn when the CH3CH2CH2CH2-OH react with excess of oxidizing agent.
Concept Introduction:
A primary alcohol gives
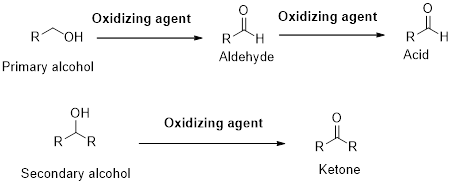
(b)
Interpretation:
The structure of the product has to be drawn when the 2-butanol react with excess of oxidizing agent.
Concept Introduction:
A primary alcohol gives carboxylic acid (RCOOH) in presence of excess oxidizing agent. However, a secondary alcohol gives ketone in presence of excess oxidizing agent. A tertiary alcohol does not undergo oxidation reaction.

(c)
Interpretation:
The structure of the product has to be drawn when the 2-methyl-2-propanol react with excess of oxidizing agent (a) CH3CH2CH2CH2-OH
Concept Introduction:
A primary alcohol gives carboxylic acid (RCOOH) in presence of excess oxidizing agent. However, a secondary alcohol gives ketone in presence of excess oxidizing agent. A tertiary alcohol does not undergo oxidation reaction.

(d)
Interpretation:
The structure of the product has to be drawn when the 2-methyl-1-propanol react with excess of oxidizing agent
Concept Introduction:
A primary alcohol gives carboxylic acid (RCOOH) in presence of excess oxidizing agent. However, a secondary alcohol gives ketone in presence of excess oxidizing agent. A tertiary alcohol does not undergo oxidation reaction.

Trending nowThis is a popular solution!

Chapter 23 Solutions
Chemistry & Chemical Reactivity
- Please see photoarrow_forward=Naming benzene derivatives Name these organic compounds: structure C1 CH3 name ☐ CH3 ப C1 × ☐arrow_forwardBlocking Group are use to put 2 large sterically repulsive group ortho. Show the correct sequence toconnect the reagent to product with the highest yield possible. * see image **NOTE: The compound on the left is the starting point, and the compound on the right is the final product. Please show the steps in between to get from start to final, please. These are not two different compounds that need to be worked.arrow_forward
- Nucleophilic Aromatic Substitution: What is the product of the reaction? What is the name of the intermediate complex? *See imagearrow_forwardPredict the final product. If 2 products are made, list which should be “major” and “minor” *see attachedarrow_forwardNucleophilic Aromatic Substitution: What is the product of the reaction? *see imagearrow_forward
- Show the correct sequence to connect the reagent to product. * see imagearrow_forwardThe answer here says that F and K have a singlet and a doublet. The singlet and doublet are referring to the H's 1 carbon away from the carbon attached to the OH. Why don't the H's two carbons away, the ones on the cyclohexane ring, cause more peaks on the signal?arrow_forwardDraw the Birch Reduction for this aromatic compound and include electron withdrawing groups and electron donating groups. *See attachedarrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
