
Concept explainers
Interpretation:
The structures with systematic names of alcohol A and acid B has to be given.
Concept introduction:
Any organic molecule can be named by using certain rules given by IUPAC (International Union for Pure and applied chemistry).IUPAC name consists of three parts, namely Prefix, suffix and root word.
Prefix- Represents the substituent present in the molecule and its position in the root name.
Suffix- Denotes the presence of
Root word - Represents the longest continuous carbon skeleton of the organic molecule.
Oxidation reaction:
Alcohol undergoes oxidation reaction using oxidising agent like KMnO4or K2Cr2O7 which yields the corresponding carbonyl compounds. Primary alcohol undergoes oxidation reaction yields the corresponding
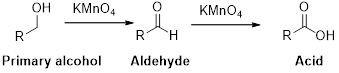
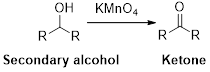
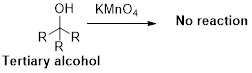
Trending nowThis is a popular solution!

Chapter 23 Solutions
Chemistry & Chemical Reactivity
- Consider this IR and NMR: INFRARED SPECTRUM TRANSMITTANCE 0.8- 0.6 0.4 0.2 3000 10 9 8 00 HSP-00-541 7 CO 6 2000 Wavenumber (cm-1) сл 5 ppm 4 M Which compound gave rise to these spectra? N 1000 1 0arrow_forwardConsider this reaction (molecular weights are under each compound): HC=CH + 2 HCI --> C2H4Cl 2 MW = 26 36.5 99 If 4.4 g of HC=CH are reacted with 110 mL of a 2.3 M HCI solution, and 6.0 g of product are actually produced, what is the percent yield?arrow_forwardWhat is the name of the major product of this reaction? OH CH3 H₂SO4, heat 1-methylcyclohexene O2-methyl-1-cyclohexene O 3-mthylcyclohexene 1-methyl-2-cyclohexenearrow_forward
- We added a brown solution of Br2 to one of our products, and the brown color disappeared. This indicated that our product wasarrow_forwardRank the following according to reactivity toward nitration: a) benzene b) bromobenzene c) nitrobenzene d) phenol Od) greatest, c) least Od) greatest, b) least Od) greatest, a) least a) greatest, b) least a) greatest, c) least Oa) greatest, d) least Ob) greatest, a) least O b) greatest, c) least Ob) greatest, d) least O c) greatest, a) least O c) greatest, b) least O c) greatest, d) leastarrow_forwardO-Nitrophenol was distilled over with the steam in our experiment while the other isomer did not. This is due to: O intramolecular hydrogen bonding in the ortho isomer O intermolecular hydrogen bonding in the the ortho isomer O the ortho isomer has a lower density O the ortho isomer has a lower molecular weightarrow_forward
- K 44% Problem 68 of 15 Submit Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. :6: :: :CI: CI CI: :0:0 Select to Add Arrows Select to Add Arrows H H Cl CI: CI CI: Select to Add Arrows Select to Add Arrows H :CI: Alarrow_forwardI I H :0: Submit Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. 0:0 :0: CI ΑΙ :CI: :CI: :0: CI Select to Add Arrows Select to Add Arrows cl. :0: Cl © ハ CI:: CI H CO Select to Add Arrows Select to Add Arrows 10: AI ::arrow_forwardOrder the following compounds from slowest to fastest in a nucleophilic acyl substitution reaction. ii 요 OB D A E C OCE Darrow_forward
- I need the most help figuring out how to find [I^-] mol/ L, [S2O8^2-] mol/L. 1st and 2nd Blank columns.arrow_forwardCan someone help me whats the issue?arrow_forwarda. The change in the Gibbs energy of a certain constant pressure process is found to fit the expression: AG-85.1 J mol −1 +36.5 J mol ¹K-1 × T A. Calculate the value of AS for the process. B. Next, use the Gibbs-Helmholtz equation: (a(AG/T)) ΔΗ - T2 to calculate the value of AH for the process.arrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning





