
Concept explainers
For alanine,
(a) 2.00.
(b) 6.00.
(c) 10.50.
What is the principal species at each pH?
(a)
Interpretation:
The value of
Concept introduction:
General formula of alanine is shown as follows:
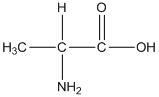
The
Answer to Problem 29QAP
The principal species at
Explanation of Solution
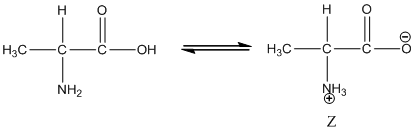
Under equilibrium condition, the Zwitter ionic form of alanine is shown as follows:
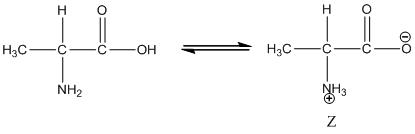
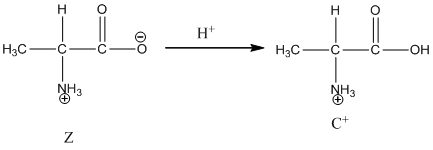
Under acidic condition, oxygen atom accepts the proton Zwitter ion exists in cationic form.
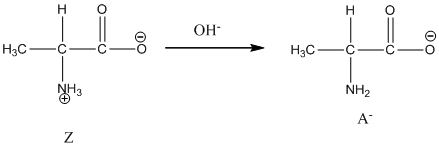
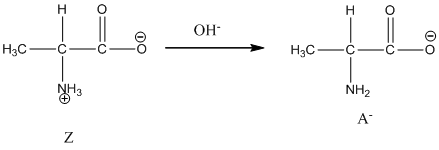
Under basic condition, base abstracts proton form nitrogen atom and it forms the anionic form.
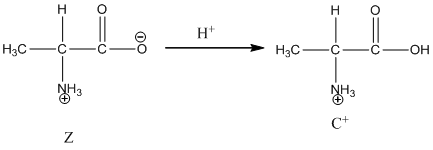
Dissociation of cation is represented as follows:
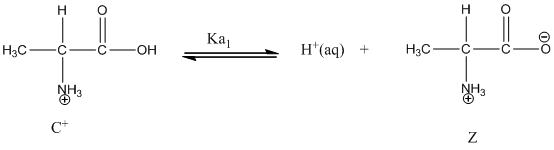
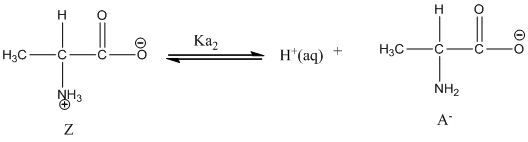
Dissociation of anion is represented as follows:
Given value of
At
So,
Take antilog both sides,
Here, Z is Zwitter ion and C is cationic form of Zwitter ion.
Here, Z is Zwitter ion and A is anionic form of Zwitter ion.
Therefore,
The principal species at
(b)
Interpretation:
The value of
Concept introduction:
General formula of alanine is shown as follows:
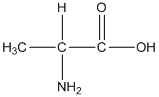
The
Answer to Problem 29QAP
The principal species at
Explanation of Solution
Under equilibrium condition zwitter ionic form of alanine is:
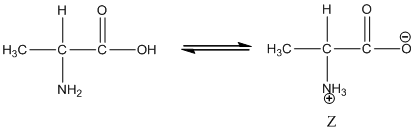
Under acidic condition oxygen atom accepts the proton zwitter ion exists in cationic form.
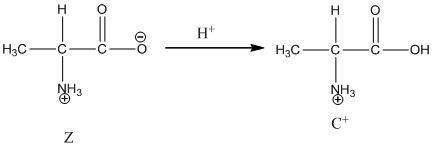
Under basic condition base abstracts proton form nitrogen atom and it forms the anionic form.
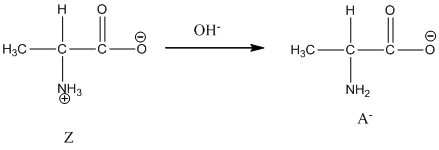
Dissociation of cation is-
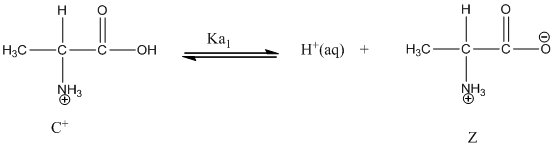
Dissociation of anion is-
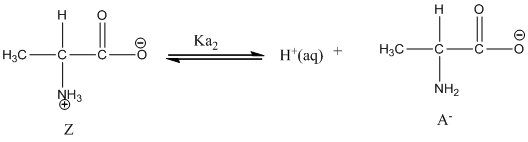
Given value of
At
So,
Take antilog both sides,
Here, Z is zwitter ion and C is cationic form of zwitter ion.
Here, Z is zwitter ion and A is anionic form of zwitter ion.
The principal species at
(c)
Interpretation:
The value of
Concept introduction:
General formula of alanine is shown as follows:
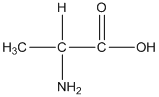
The
Answer to Problem 29QAP
The principal species at
Explanation of Solution
Under equilibrium condition zwitter ionic form of alanine is:
Under acidic condition oxygen atom accepts the proton zwitter ion exists in cationic form.
Under basic condition base abstracts proton form nitrogen atom and it forms the anionic form.
Dissociation of cation is-
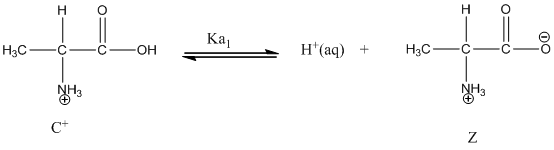
Dissociation of anion is-
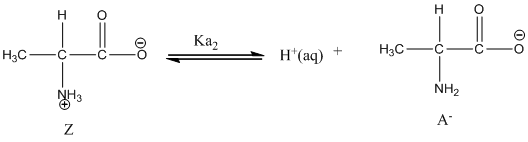
Given value of
At
So,
Take antilog both sides,
Here, Z is zwitter ion and C is cationic form of zwitter ion.
Here, Z is zwitter ion and A is anionic form of zwitter ion.
The principal species at
Want to see more full solutions like this?
Chapter 23 Solutions
Chemistry: Principles and Reactions
- Calculate the following values. Enter your answers in scientific notation. (a) What is the Kb of the benzoate ion, C6H5COO−? Kb = × 10 (b) What is the Ka of the 2−hydroxyethylammonium ion, HOCH2CH2NH3+? ( pKb of HOCH2CH2NH2 = 4.49 ) Ka = × 10arrow_forwardThe base methylamine ( CH3NH₂) has a Kb of 5.0 x 10-4. A closely related base, trimethylamine ( (CH3)3N), has a Kb of 7.4 x 10-5. a Which of the two bases is stronger? methylamine trimethylamine Correct Since methylamine has the larger K₁, methylamine is the stronger base. b Calculate the pH of a 0.38 M solution of the stronger base. pH =arrow_forwardAlanine is a diprotic amino acid with a pKa = 2.344 for the carboxylic group and a pKa = 9.868 for the ammonium group. Estimate the pH of a solution of 0.340 M alanine. Answer using two significant figures.arrow_forward
- Calculate the pH of a 0.324 M aqueous solution of diethylamine ((C₂H5)₂NH, K₁ = 6.9×10-4). pH =arrow_forwardQ1. The pKa values of Glutamic acid are 2.2, 4.3 and 9.7. H2N HO. OH (a) Calculate the pH of a glutamic acid solution in which the a-amine group is 80% deprotonated? (show your calculation!) (b) Draw the ionic forms of glutamic acid that exist at the calculated pH.arrow_forwardYou are given the structures of the amino acids alanine (Ala), methionine (Met) and threonine (Thr). to H2N CO2H H2N CO2H H2N co,Harrow_forward
- How is SO3 more acidic than MgO? SO3 + H2O = H2SO4 so it can give two (H+) but MgO + H2O = Mg(OH)2, wouldn't it therefore also be able to give 2 (H+) making it just as acidic as SO3? can someone explain?arrow_forwardAbbreviating malonic acid, CH2(CO2H)2, as H2A (pK1 = 2.847, pK2 = 5.696), find the pH of 0.100 M solution of NaHA.arrow_forwardBelow is the amino acid L-cysteine. Please draw a peptide chain consisting of 4 cysteines (don't worry about stereochemistry). H₂N SH O Oarrow_forward
- (a) Given that Kb for ammonia is 1.8 x10-5 and that forhydroxylamine is 1.1 x 10-8, which is the stronger base?(b) Which is the stronger acid, the ammonium ion or thehydroxylammonium ion? (c) Calculate Ka values for NH4+and H3NOH+.arrow_forwardWhat is the conjugate acid of H2C6H7O5 -1aq2? What is its conjugate base?arrow_forwardCalculate the pH of a 0.020 M solution of phenylacetic acid, C6H5CH2COOH. What will be the pH if the solution is made 0.050 M with its sodium salt, C6H5CH2COONa? Ka= 4.9 x 10-5.arrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





