
Concept explainers
Interpretation:
The two amino acids in the given compound Aspartame should be identified and all the product of digestion of this compound has to be determined.
Concept introduction:
Amino acids are the molecules containing an
Carbon, hydrogen, oxygen and nitrogen are the key elements in amino acid.
General structure of an amino acid can be drawn as follows,
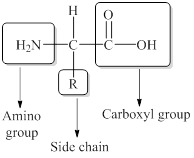
The amino acids are distinguished from one another by their R group also known as a side chain.
Some amino acids are listed below,
Peptide is a short
Peptide bonds are formed by a condensation reaction of carboxylic group of an amino acid and amino group of another amino acid with the removal of water molecule.
Hydrolysis will result in peptide being reduced to simpler amino acids.
Want to see the full answer?
Check out a sample textbook solution
Chapter 23 Solutions
General Chemistry: Atoms First
- Fats belong to the class of organic compounds represented by the general formula, RCOOR', where R and R' represent hydrocarbon groups. What is the name of the functional group present in fats? What functional group is common to all saponifiable lipids?arrow_forwardWhich of the following are true concerning the chemical bond that forms between the carboxyl (RCOOH) group of one amino acid and the amino (RCNH2) group of another? a.The bond is called a peptide bond. b.It is formed by inserting a water molecule between them. c.It is formed by a dehydration reaction. d.A polypeptide has more of these bonds than a protein.arrow_forwardFill in the blanks in the following statements: aThe order of the amino acids in a protein is the ______ structure of that protein. The _______ of the backbone chain of a protein is the secondary structure of that protein. The tertiary structure of a protein describes the ______ of the secondary structure. b Hydrogen bonding between the oxygen atoms of carbonyl groups and the hydrogen atoms of amide groups in the same protein chain gives the secondary structure, called the ______. c Hydrogen bonding between the oxygen atoms of carbonyl groups and the hydrogen atoms of amide groups in the adjacent protein chain gives the secondary structure, called the ______.arrow_forward
- 21-90 To what extent do lipids and carbohydrates play structural roles in living organisms? Do these roles differ in plants and in animals?arrow_forwardWhat special role does the amino acid cysteine have in the peptides vasopressin and oxytocin?arrow_forward"Lipids are biomolecules that are soluble in organic solvents andinsoluble in water" Define this ?arrow_forward
- V. Answer the following questions in not more than two sentences. 1. What is the function of lipids in detergents or soaps? 2. What intermolecular forces stabilize the double helix structure of DNA? 3. The primary structure of a protein is maintained by peptide bonds. Are peptide bonds intramolecular or intermolecular attraction? Why do you say so? Answers here:arrow_forwardGive an example of (a) polyester (b) polyamide.arrow_forwardExplain the organic compound- capsaicin ?arrow_forward
- Amino acids can be synthesized by reductive amination. Draw the structure of the organic compound that you would use to synthesize aspartic acid.arrow_forwardWrite the structure of the given peptide: • threonyalanyltyrosine (A tripeptide made up of the amino acids: threonine, alanine and tyrosine)arrow_forwardPart 4 - Lipids The cow milk B-lactoglobulin protein can bind to several lipids among which myristic acid, palmitic acid, stearic acid, and oleic acid. Question 4-1 b) Draw the semi-developed formula of both the palmitic acid and the oleic acid at physiological pH. Circle the hydrophilic part of each molecule. Do not forget to number the carbon atoms.arrow_forward
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning





