
(a) Define carbocation.
(b) Which of the following are carbocations?
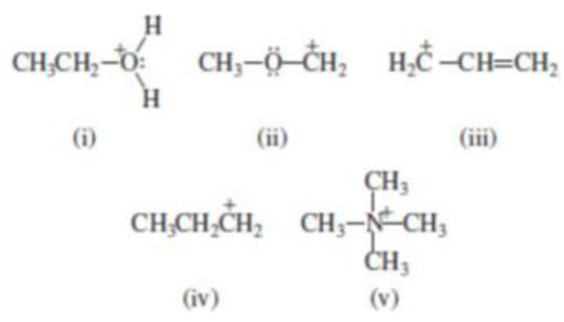
(i)
Interpretation:
Explanation of carbocation, Carbocation should be identified for the given molecule.
Concept introduction:
Carbocation: carbon atom bears positive charged species with three bonds is called Carbocation and it plays vital intermediate in organic synthesis. Carbocation migratory aptitude is mainly depends on the stability of the carbocation.
Carbocation’s are classified in to three types as primary, secondary and tertiary depending on the number of carbon atoms which is attached to the ionized carbon.
Tertiary carbocation is more stable than secondary carbocation, secondary carbocation is more stable than the primary carbocation.
Answer to Problem 23.53QP
Answer
Carbocation: carbon atom bears positive charged species with three bonds is called Carbocation and it plays vital intermediate in organic synthesis. Carbocation migratory aptitude is mainly depends on the stability of the carbocation. (a).
Explanation of Solution
To explain:carbocation
Structure of the carbocation is given below,
Carbon atom bears positive charged species with three bonds is called Carbocation and it plays vital intermediate in organic synthesis. Carbocation migratory aptitude is mainly depends on the stability of the carbocation.
(ii)
Interpretation:
Explanation of carbocation, Carbocation should be identified for the given molecule.
Concept introduction:
Carbocation: carbon atom bears positive charged species with three bonds is called Carbocation and it plays vital intermediate in organic synthesis. Carbocation migratory aptitude is mainly depends on the stability of the carbocation.
Carbocation’s are classified in to three types as primary, secondary and tertiary depending on the number of carbon atoms which is attached to the ionized carbon.
Tertiary carbocation is more stable than secondary carbocation, secondary carbocation is more stable than the primary carbocation.
Answer to Problem 23.53QP
Answer
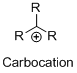
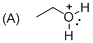
The given molecule is not a carbocation, the structure of the molecule is shown below
Explanation of Solution
To find: The carbocation.
The given molecule is not a carbocation, the structure of the molecule is shown below
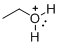
Oxygen is bearing positive charge, so the given molecule is not a carbocation.
(iii)
Interpretation:
Explanation of carbocation, Carbocation should be identified for the given molecule.
Concept introduction:
Carbocation: carbon atom bears positive charged species with three bonds is called Carbocation and it plays vital intermediate in organic synthesis. Carbocation migratory aptitude is mainly depends on the stability of the carbocation.
Carbocation’s are classified in to three types as primary, secondary and tertiary depending on the number of carbon atoms which is attached to the ionized carbon.
Tertiary carbocation is more stable than secondary carbocation, secondary carbocation is more stable than the primary carbocation.
Answer to Problem 23.53QP
Answer
The given molecule is carbocation, the structure of the molecule is shown below
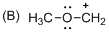
Explanation of Solution
To find: The carbocation.
The given molecule is carbocation, the structure of the molecule is shown below
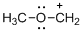
Carbon atom bears positive charged species with three bonds so the given molecule is called Carbocation
(vi)
Interpretation:
Explanation of carbocation, Carbocation should be identified for the given molecule.
Concept introduction:
Carbocation: carbon atom bears positive charged species with three bonds is called Carbocation and it plays vital intermediate in organic synthesis. Carbocation migratory aptitude is mainly depends on the stability of the carbocation.
Carbocation’s are classified in to three types as primary, secondary and tertiary depending on the number of carbon atoms which is attached to the ionized carbon.
Tertiary carbocation is more stable than secondary carbocation, secondary carbocation is more stable than the primary carbocation.
Answer to Problem 23.53QP
Answer
The given molecule is carbocation, the structure of the molecule is shown below (d)
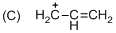
Explanation of Solution
To find:The carbocation.
The given molecule is carbocation, the structure of the molecule is shown below
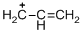
Carbon atom bears positive charged species with three bonds so the given molecule is called Carbocation
(v)
Interpretation:
Explanation of carbocation, Carbocation should be identified for the given molecule.
Concept introduction:
Carbocation: carbon atom bears positive charged species with three bonds is called Carbocation and it plays vital intermediate in organic synthesis. Carbocation migratory aptitude is mainly depends on the stability of the carbocation.
Carbocation’s are classified in to three types as primary, secondary and tertiary depending on the number of carbon atoms which is attached to the ionized carbon.
Tertiary carbocation is more stable than secondary carbocation, secondary carbocation is more stable than the primary carbocation.
Answer to Problem 23.53QP
Answer
The given molecule is carbocation, the structure of the molecule is shown below (e)
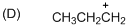
Explanation of Solution
To find:The carbocation.
The given molecule is carbocation, the structure of the molecule is shown below
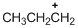
Carbon atom bears positive charged species with three bonds so the given molecule is called Carbocation.
(vi)
Interpretation:
Explanation of carbocation, Carbocation should be identified for the given molecule.
Concept introduction:
Carbocation: carbon atom bears positive charged species with three bonds is called Carbocation and it plays vital intermediate in organic synthesis. Carbocation migratory aptitude is mainly depends on the stability of the carbocation.
Carbocation’s are classified in to three types as primary, secondary and tertiary depending on the number of carbon atoms which is attached to the ionized carbon.
Tertiary carbocation is more stable than secondary carbocation, secondary carbocation is more stable than the primary carbocation.
Answer to Problem 23.53QP
Answer
The given molecule is not a carbocation, the structure of the molecule is shown below (f)
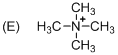
Explanation of Solution
To find: The carbocation.
The given molecule is not a carbocation, the structure of the molecule is shown below (f)
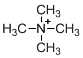
Nitrogen is bearing positive charge with four bonds, so the given molecule is not a carbocation.
Want to see more full solutions like this?
Chapter 23 Solutions
Chemistry: Atoms First
- Indicate whether each statement is true or false. (a) Pentanehas a higher molar mass than hexane. (b) The longer the linearalkyl chain for straight-chain hydrocarbons, the higherthe boiling point. (c) The local geometry around the alkynegroup is linear. (d) Propane has two structural isomers.arrow_forwardAn alkane, P, has the molecular formula, C,H.. An alkene, Q, has the molecular formula, C H,. (a) Name P and Q ánd write their full structural formulae. (b) State two differences between P and Q in terms of their structures. x'arrow_forwardWrite structural formulas for compounds that meet the following descriptions:(a) An alkene, C6H12, that cannot have cis–trans isomersand whose longest chain is 5 carbons long(b) An alkene with a chemical formula of C10H12 that hascis–trans isomers and contains a benzene ring.arrow_forward
- Draw the structures of the following compounds. (a) 1-chloro-1 isopropylcyclopentanearrow_forward(a) Draw the four isomers of C₅H₁₀O that can be oxidizedto an aldehyde. (b) Draw the three isomers of C₅H₁₀O that canbe oxidized to a ketone. (c) Draw the isomers of C₅H₁₀O that cannot be easily oxidized to an aldehyde or ketone. (d) Name any isomer that is an alcohol.arrow_forwardIndicate whether each statement is true or false. (a) Twogeometric isomers of pentane are n-pentane and neopentane.(b) Alkenes can have cis and trans isomers around theCC double bond. (c) Alkynes can have cis and trans isomersaround the CC triple bond.arrow_forward
- 1. (a) Draw the structures of the eight isomeric pentyl alcohols, C3H11OH. (b) Name each by the IUPAC system and by the carbinol system. (c) Label each as primary, secondary, or tertiary, (d) Which one is isopentyl alcohol? tert-Pentyl alcohol? (e) Give the structure of a primary, a secondary, and a tertiary alcohol of the formula C,H13OH.arrow_forward(a) What is the molecular formula of hexane, the alkane withsix carbonsarrow_forwardCyclopropane (C3H6, a three-membered ring) is more reactive than most other cycloalkanes.(a) Draw a Lewis structure for cyclopropane.(b) Compare the bond angles of the carbon atoms in cyclopropane with those in an acyclic (noncyclic) alkane.(c) Suggest why cyclopropane is so reactive.arrow_forward
- (b) Draw the structural formula for each of the following compounds. (i) 2,2,3-trimethylpentan-3-ol(ii) 2-methyl-3-phenylbut-2-enal(iii) 5-chloro-2-methylheptan-3-olarrow_forward(a) Write an equation involving structural formulas forthe catalytic cracking of 2,2,3,4,5,5-hexamethylhexane. Assume that the cracking occurs between carbon atoms 3 and 4.(b) Draw and name one other isomer of the alkene.arrow_forwardA certain hydrocarbon has a molecular formula of C5H8. Which of the following is not a structural possibility for this hydrocarbon? (a) It is a cycloalkane. (b) It contains one ring and one double bond. (c) It contains two double bonds and no rings. (d) It is an alkyne.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





