
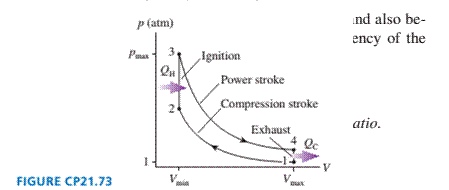
The gasoline engine in your car can be modeled as the Otto
cycle shown in FIGURE CP21.73. A fuel-air mixture is sprayed into
the cylinder at point l, where the piston is at its farthest distance
from the spark plug. This mixture is compressed as the piston
moves toward the spark plug during the adiabatic compression
stroke. The spark plug fires at point 2, releasing heat energy that
had been stored in the gasoline. The fuel burns so quickly that the
piston doesn't have time to move, so the heating is an isochoric
process. The hot, high-pressure gas then pushes the piston out-
ward during the power stroke. Finally, an exhaust value opens to
allow the gas temperature and pressure to drop back to their initial
values before starting the cycle over again.


Trending nowThis is a popular solution!

Chapter 21 Solutions
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
- Consider these scenarios and state whether work is done by the system on the environment (SE) or by the environment on the system (ES): (a) opening a carbonated beverage; (b) filling a flat tire; (c) a sealed empty gas can expands on a hot day, bowing out the walls.arrow_forwardCalculate the net work output of a heat engine following path ABCDA in the figure below, where V₁ = 10.6 × 10-³ m³ and V/₂ = 42.4 x 10-³3 m³. How is the work done measured for an enclosed loop in the case of a heat engine? J P (106 N/m²) 2.6 2.0 1.0 0.6 !₁ I I A D V₁ V (10-³ m³) I B V₂arrow_forwardA Carnot heat engine operates with an efficiency η=0.5 between a hot reservoir at temperature T1 and a cold reservoir at temperature T2=300 K. Calculate the temperature T1 of the hot reservoir. Select one: a.T1=150.0 b.T1=300.0 c.T1=900.0 d.T1=600.0arrow_forward
- In1816, Robert Stirling, a Scottish clergyman , patented the Stirling engine which has found a wide variety of applications ever since, including current use in solar energy collectors to transform sunlight into electricity. Fuel is burned externally to warm one of the engine's two cylinders, A fixed quantity of inert gas moves cyclically between the cylinders, expanding in the hot one and contracting in the cold one. Figure P21.33 represents a model for its thermodynamic cycle. Consider n moles of an ideal monoatomic gas being taken once through the cycle, consisting of two isothermal processes at temperatures 3Tiand Ti and two constant-volume processes. Let us find the efficiency of this engine .(a) Find the energy transferred by heat into gas during the isovolumetric process AB. (b) Find the energy transferred by heat into the gas during the isothermal process BC. (c) Find the energy transferred by heat into the gas during isovolumetric process CD. (d) Find the enrgy transferred by…arrow_forwardA Carnot engine operates between the temperatures Th = 1.00 x 102°C and Tc = 20.0°C. By what factor does the theoretical efficiency increase if the temperature of the hot reservoir is increased to 5.50 x 102°C?arrow_forwardTo improve the efficiency of a Carnot engine, you can increase the difference in temperature between its cold and hot reservoir. You have a Carnot engine that usually operates between 710.5 K (hot) and 444.5 K (cold). You can apply a change of temperature of 42 K to either the hot (710.5+42, case 1) or the cold (444.5-42, case 2). Calculate the ratio in efficiencies: e(case1) / e(case2).arrow_forward
- A Carnot engine operates between the temperatures Th = 100°C and Tc = 20°C. By what factor does the theoretical efficiency increase if the temperature of the hot reservoir is increased to 550°C?arrow_forwardA heat engine takes in 1800 J of heat from a high temperature reservoir and rejects 1200 J of heat to a lower temperature reservoir. How much work does the engine do in each cycle and what is its efficiency? a) W = 600 J, e = 33.3% b) W = 600 J, e = 50% c) none of these.arrow_forwardA Carnot engine has a thermal efficiency of 0.60. The temperature of the hot reservoir is 800 K and 3000 J of heat is rejected to the cold reservoir. The temperature of the cold reservoir isarrow_forward
- Find the work done by the engine cycle shown in the P-V diagram below. P(kPa) 4 2 Q23 3 200 - Q12 Work Q 34 100- 1 Q41 V(L) + 5.0 + 20 а. 1.0 kJ b. 2.5 kJ C. 1.5 kJ d. 3.0 kJ е. 2.0 kJarrow_forwardA Carnot engine operates between the temperatures Th = 100°C and Tc = 21°C. By what factor does the theoretical efficiency increase if the temperature of the hot reservoir is increased to 465°C? ec, new ec =arrow_forward
 Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning



