
a)
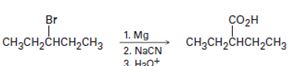
Interpretation:
The flaw in the synthetic scheme given is to be identified.
Concept introduction:
To identify:
The flaw in the synthetic scheme given.
b)
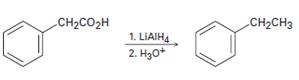
Interpretation:
The flaw in the synthetic scheme given is to be identified.
Concept introduction:
Carboxylic acids are reduced to alcohols and not to
To identify:
The flaw in the synthetic scheme given.
c)
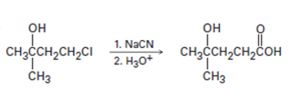
Interpretation:
The flaw in the synthetic scheme given is to be identified.
Concept introduction:
Alkyl halides when treated with cyanides yield nitriles which upon hydrolysis give carboxylic acids. Tertiary alcohols get dehydrate to yield
To identify:
The flaw in the synthetic scheme given.
Trending nowThis is a popular solution!

Chapter 20 Solutions
Organic Chemistry
- Hi!! Please provide a solution that is handwritten. Ensure all figures, reaction mechanisms (with arrows and lone pairs please!!), and structures are clearly drawn to illustrate the synthesis of the product as per the standards of a third year organic chemistry course. ****the solution must include all steps, mechanisms, and intermediate structures as required. Please hand-draw the mechanisms and structures to support your explanation. Don’t give me AI-generated diagrams or text-based explanations, no wordy explanations on how to draw the structures I need help with the exact mechanism hand drawn by you!!! I am reposting this—ensure all parts of the question are straightforward and clear or please let another expert handle it thanks!!arrow_forwardIn three dimensions, explain the concept of the velocity distribution function of particles within the kinetic theory of gases.arrow_forwardIn the kinetic theory of gases, explain the concept of the velocity distribution function of particles in space.arrow_forward
- In the kinetic theory of gases, explain the concept of the velocity distribution function of particles.arrow_forwardHi!! Please provide a solution that is handwritten. this is an inorganic chemistry question please answer accordindly!! its just one question with parts JUST ONE QUESTION with its parts spread out till part (g), please answer EACH part till the end and dont just provide wordy explanations wherever asked for structures, please DRAW DRAW them on a paper and post clearly!! answer the full question with all calculations step by step EACH PART CLEARLY please thanks!! im reposting this please solve all parts and drawit not just word explanations!!arrow_forwardHi!! Please provide a solution that is handwritten. this is an inorganic chemistry question please answer accordindly!! its just one question with parts JUST ONE QUESTION, please answer EACH part PART A AND PART B!!!!! till the end and dont just provide wordy explanations wherever asked for structures, please DRAW DRAW them on a paper and post clearly!! answer the full question with all details EACH PART CLEARLY please thanks!! im reposting this please solve all parts and drawit not just word explanations!!arrow_forward
- Hi!! Please provide a solution that is handwritten. this is an inorganic chemistry question please answer accordindly!! its just one question with parts JUST ONE QUESTION, please answer EACH part till the end and dont just provide wordy explanations wherever asked for structures, please DRAW DRAW them on a paper and post clearly!! answer the full question with all details EACH PART CLEARLY please thanks!! im reposting this please solve all parts and drawit not just word explanations!!arrow_forward8b. Explain, using key intermediates, why the above two products are formed instead of the 1,2-and 1,4- products shown in the reaction below. CIarrow_forward(5pts) Provide the complete arrow pushing mechanism for the chemical transformation depicted below Use proper curved arrow notation that explicitly illustrates all bonds being broken, and all bonds formed in the transformation. Also, be sure to include all lone pairs and formal charges on all atoms involved in the flow of electrons. CH3O H I I CH3O-H H I ① Harrow_forward

 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


