
Essential Organic Chemistry, Global Edition
3rd Edition
ISBN: 9781292089034
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 20.6, Problem 12P
Interpretation Introduction
Interpretation:
The two ways that isoprene linkages can be linked head-to-tail to form menthol has to be determined.
Concept Introduction:
Terpenes are made by joining five-carbon units, usually in a head to tail-fashion.
Monoterpenes are those terpenes with two isoprene units-have 10 carbons, sesquiterpenes have 15 carbons, diterpenes have 20 carbons, triterpenes have 30 carbons and tetraterpenes have 40 carbons.
Isopentenyl pyrophosphate is the five-carbon compound used for the biosynthesis of terpenes.
Isoprene unit:
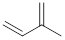
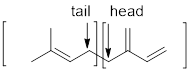
Branched end of isoprene – Head
Unbranched end of isoprene - Tail
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
If this structure were to go through a dehydration reaction, what would be the different isomers that can be made? I can only think of one structure with the double bond.
The saponification of triglycerides and its reverse reaction of the condensation of fatty acids and
glycerol to make a triglyceride is just one example of how carbonyl chemistry is important in living
organisms. List down two other classes of biomolecules that rely on carbonyls for part of their chemical
characteristics and illustrate a simple representative structure of that biomolecule, identifying where
the carbonyl is.
Write the Structure Activity Relationship (SAR) of 1,4-Dihydropyridine? Please answer at your own words.
Chapter 20 Solutions
Essential Organic Chemistry, Global Edition
Ch. 20.1 - Prob. 1PCh. 20.2 - Prob. 2PCh. 20.2 - Prob. 3PCh. 20.2 - Draw the structure of an optically active fat...Ch. 20.4 - Prob. 6PCh. 20.4 - Prob. 7PCh. 20.4 - The membrane phospholipids in deer have a higher...Ch. 20.4 - Prob. 9PCh. 20.6 - Prob. 10PCh. 20.6 - Prob. 11P
Ch. 20.6 - Prob. 12PCh. 20.7 - Propose a mechanism for the biosynthesis of...Ch. 20.7 - Prob. 14PCh. 20.8 - Draw the individual 1,2-hydride and 1,2-methyl...Ch. 20.9 - Prob. 16PCh. 20 - Prob. 17PCh. 20 - Prob. 18PCh. 20 - Cardiolipins are found in heart muscles. Draw the...Ch. 20 - Prob. 20PCh. 20 - 5-Androstene-3,17-dione is isomerized to...Ch. 20 - Prob. 22PCh. 20 - Prob. 23PCh. 20 - Prob. 24PCh. 20 - Eudesmol is a sesquiterpene found in eucalyptus....
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Considering the structure of the hydrocarbon test samples (benzene, cyclohexane, toluene, heptane, cyclohexane, hexane), which among the test samples will react with the nitrating mixture?arrow_forwardThe monomers -(CH2)-C-OH and H2N- -NH2 но- would react with each other to form a polycarbonate a polyamide a polyesterarrow_forwardWhen the conjugate acid of aniline, C6H5NH3+, reacts with the acetate ion, the following reaction takes place: C6H5NH3+(aq)+CH3COO(aq)C6H5NH2(aq)+CH3COOH(aq) If Kafor C6H5NH3+ is 1.35105 and Kafor CH3COOH is 1.86105 , what is K for the reaction?arrow_forward
- What alcohol is formed by the addition of water in the presence of sulfuric acid (an addition reaction) to 1-pentene?arrow_forwardChoose one test that can be applied on hydroxybenzene and heptane; cyclohexane and cyclohexene. Solubility Test Litmus Test Hydrocarbon Saturation (Bromination, Baeyer's test) Oxidation Test for Alcohol (KMnO4, K2CrO7) Lucas Test FeCl3 Test DNP Test Schiff's Test Tollen's Test Iodoform Test Ester test Nitrous Acid Testarrow_forwardWhat are tautomers ?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning