
a)
Interpretation : Oxidizing and reducing agents in the
Concept Introduction: The chemical reaction in which oxidation and reduction take place simultaneously is known as
a)
Answer to Problem 15SP
The nitrogen atom of
Explanation of Solution
Oxidation is the removal of an electron and the species that undergo oxidation and reduce another species is known as the reducing agent. Similarly, the reduction is the addition of an electron or oxygen atom, and the species that undergo reduction by oxidizing other species is known as a reducing agent.
The given reaction,
The reaction can also be written as,
The oxidation number of Nitrogen is calculated as follows:
So, let the oxidation number of the nitrogen atom of
So, let the oxidation number of the nitrogen atom of
The oxidation number of nitrogen in
is calculated as follows:
Let the oxidation number of the nitrogen atom of
In water molecules, the charge of the hydrogen atom is
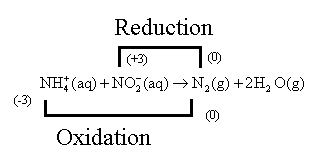
Thus, from the reaction, it is concluded that the Nitrogen atom of
b)
Interpretation : Oxidizing and reducing agents in the chemical reaction are to be identified.
Concept Introduction: The chemical reaction in which oxidation and reduction take place simultaneously is known as a redox reaction.
b)
Answer to Problem 15SP
The lead atom of
Explanation of Solution
Oxidation is the removal of an electron and reduction is the addition of an electron or oxygen atom.
The given reaction,
The reaction can also be written as,
The oxidation number of lead is calculated as follows:
So, let the oxidation number of the lead atom be
So, let the oxidation number of the iodine atom of
The oxidation number of nitrogen in
is calculated as follows:
Let the oxidation number of the nitrogen atom of
The oxidation number of lead in
is calculated as follows:
Let the oxidation number of the nitrogen atom of
In water molecules, the charge of the hydrogen atom is
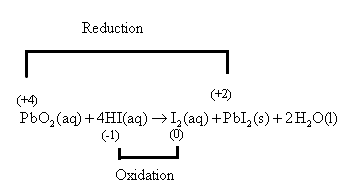
Hence, the Lead atom of
Chapter 20 Solutions
Chemistry 2012 Student Edition (hard Cover) Grade 11
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





