
Organic Chemistry (6th Edition)
6th Edition
ISBN: 9781260119107
Author: Janice Gorzynski Smith
Publisher: McGraw Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 20, Problem 56P
What
a. 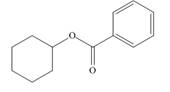 b.
b. 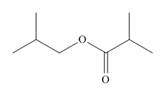
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
2. What carboxylic acid and alcohol are needed to prepare each ester by Fischer esterification?
a)
b)
c)
Which compound in each pair has the higher boiling point?
Он
a.
or
b.
or
A.
Which of the following are a. hemiacetals? b. acetals? c. hydrates?
Chapter 20 Solutions
Organic Chemistry (6th Edition)
Ch. 20.1 - Prob. 1PCh. 20.2 - Draw the three possible resonance structures for...Ch. 20.2 - Prob. 3PCh. 20.3 - Give an IUPAC or common name for each compound. a....Ch. 20.3 - Problem 22.5 Draw the structure corresponding to...Ch. 20.4 - Problem 22.6 Explain why the boiling point of is...Ch. 20.7 - Prob. 11PCh. 20.7 - Prob. 12PCh. 20.8 - Prob. 13PCh. 20.9 - Problem 22.16 Draw the products of each reaction.
...
Ch. 20.9 - Prob. 17PCh. 20.9 - Problem 22.18 Draw a stepwise mechanism for the...Ch. 20.9 - Prob. 19PCh. 20.10 - Problem 22.20 Fenofibrate is a...Ch. 20.10 - Problem 22.21 What product is formed when the...Ch. 20 - Prob. 33PCh. 20 - 22.40 Give the IUPAC or common name for each...Ch. 20 - 22.41 Give the structure corresponding to each...Ch. 20 - Prob. 36PCh. 20 - 22.43 Explain why is a stronger acid and a weaker...Ch. 20 - (a) Propose an explanation for the difference in...Ch. 20 - Draw the product formed when phenylacetic acid is...Ch. 20 - Prob. 42PCh. 20 - Prob. 43PCh. 20 - Prob. 44PCh. 20 - Prob. 45PCh. 20 - Prob. 46PCh. 20 - Prob. 47PCh. 20 - Prob. 48PCh. 20 - 22.64 What carboxylic acid and alcohol are needed...Ch. 20 - Problem 22.65 Devise a synthesis of each compound...Ch. 20 - 22.70 What polyester or poly amide can be prepared...Ch. 20 - 22.71 What two monomers are needed to prepare each...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 17. Which functional groups are in the following molecule of aspirin? HO, a benzene ring, an ester, a ketone, and an alcohol b. a benzene ring, two ketones, an ether, and an alcohol a benzene ring, a carboxylic acid, an ether, and a ketone d. a benzene ring, a carboxylic acid, and an ester a. c.arrow_forwardB. Give the IUPAC name for each compound. a. b. d.arrow_forwardAn acid catalyst in nucleophilic addition of aldehydes and ketones is used for: Select one: a. Protonation of carbonyl carbon b. Making the aldehyde and ketone more susceptible to nucleophiles c. Increasing the nucleophilicity of the nucleophile d. To provide a medium for the reaction.arrow_forward
- 18. Identify the lactone formed by the following hydroxy carboxylic acid. A. A B. B C. C D. D OH OH & & A) B) C) D)arrow_forwardGive the IUPAC name for each sulde.arrow_forwardWhy is the water solubility of a carboxylate salt greater than that of its parent carboxylic acid? A) A carboxylate salt is an ionic compound which is soluble in water. B) A carboxylate salt is less polar than its parent carboxylic acid. C) A carboxylate salt has a lower boiling point than its parent carboxylic acid. D) A carboxylate salt is more readily reduced to an aldehyde than its parent carboxylic acid.arrow_forward
- Saponification product of butylpropanoate is: Propanol and Sodium butanoate Butanol and sodium propanoate Butanol and Propanoic acid Butanoic acid and Propanoic acidarrow_forward2. How many different ß-hydroxyaldehydes and ß-hydroxyketones, including constitutional isomers and stereoisomers, are formed upon treatment of a mixture of acetone and benzaldehyde with base? a. b. 2 c. 3 d. 4arrow_forwardCompound that is most easily hydrolyzed by acid in water `NH A. В. F C. D.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY