
Concept explainers
Interpretation:
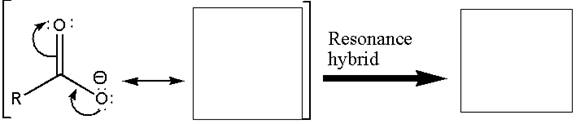
To illustrate the stabilization of a carboxylate anion, the resonance structure and resonance hybrid structure of the carboxylate ion are to be drawn.
Concept introduction:
Resonance exists in species for which there are two or more valid Lewis structures. Resonance structures differ only in the placement of their valence electrons, not their atoms. Resonance structures are imaginary; the one, true species is represented by the resonance hybrid. A resonance hybrid is a weighted average of all resonance structures. Resonance provides stabilization. The resonance hybrid looks most like the most stable resonance structure. Resonance stabilization is usually large when resonance structures are equivalent.
Want to see the full answer?
Check out a sample textbook solution
Chapter 20 Solutions
Organic Chemistry: Principles and Mechanisms (Second Edition)
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning


