
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Chapter 2, Problem 3E
(a)
Interpretation Introduction
Interpretation:
The
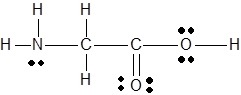
Concept Introduction :
There are three steps to determine the bond angles in a molecule.
- Lewis structure should be drawn
- The electron domain geometry should be determine using the steric number and VSEPR theory.
- Then the shape of the molecule is used to determine the angles between electron domains.
(b)
Interpretation Introduction
Interpretation:
The
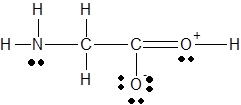
Concept Introduction :
There are three steps to determine the bond angles in a molecule.
- Lewis structure should be drawn
- The electron domain geometry should be determine using the steric number and VSEPR theory.
- Then the shape of the molecule is used to determine the angles between electron domains.
(c)
Interpretation Introduction
Interpretation:
The prediction expected to be more accurate should be explained.
Concept Introduction :
There are three steps to determine the bond angles in a molecule.
- Lewis structure should be drawn
- The electron domain geometry should be determine using the steric number and VSEPR theory.
- Then the shape of the molecule is used to determine the angles between electron domains.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
The formula for nitryl chloride is CINO2 (in which N is the central atom).
a.Draw the Lewis structure for the molecule, including all resonance structures.
b.What is the N-O bond order?
c.Describe the electron-pair and molecular geometries and give values for all bond angles.
d.What is the most polar bond in the molecule? Is the molecule polar?
e.The computer program used to calculate electrostatic potential surfaces gave the following charges on atoms in the molecule: A =-0.03, B = -0.26, and C = +0.56. Identify the atoms A, B, and C. Are these calculated charges in accord with your predictions?
Sodium Azide is an ionic compound that is used in automotive air bags. It has the chemical
formula NaN3 (The Azide ion is N , and should not be confused with the Nitride ion,
N°).
a. Give the LEWIS diagram of the Azide ion.
b. What is the geometry of the Azide ion? What is the bond angle of the Azide
ion?
How does adding an atom affect the position and angles of existing atoms or lone pairs?
How does adding a lone pair affect the position and angles of existing atoms and lone pairs?
Is the effect of adding bonded atoms and lone pairs to the central similar? Explain your answer.
Describe what is meant by the "Steric #".
Explain the difference between the terms "Electron Geometry" and "Molecule Geometry".
How does changing a bond to a double or triple bond affect the shape of the molecules?
List the molecules in Part III where the real bond angles differ from the theoretical model values. Why do you think the values differ?
Chapter 2 Solutions
Organic Chemistry: A Guided Inquiry
Ch. 2 - Prob. 1CTQCh. 2 - The valence shell of an atom in a legitimate Lewis...Ch. 2 - Prob. 3CTQCh. 2 - Prob. 4CTQCh. 2 - Prob. 5CTQCh. 2 - It is impossible to draw a legitimate Lewis...Ch. 2 - Describe how to calculate the total number of...Ch. 2 - Prob. 8CTQCh. 2 - Prob. 9CTQCh. 2 - Prob. 10CTQ
Ch. 2 - Prob. 11CTQCh. 2 - Prob. 12CTQCh. 2 - A complete Lewis structure must show all nonzero...Ch. 2 - Prob. 14CTQCh. 2 - Prob. 15CTQCh. 2 - Prob. 16CTQCh. 2 - Prob. 17CTQCh. 2 - Prob. 18CTQCh. 2 - Complete the rest of the table for N, O or X by...Ch. 2 - Prob. 20CTQCh. 2 - Prob. 21CTQCh. 2 - Make a checklist that can be used to determine if...Ch. 2 - Prob. 2ECh. 2 - Prob. 3ECh. 2 - Draw the Lewis structure of a neutral molecule...Ch. 2 - Prob. 5ECh. 2 - For each element, predict (and draw a Lewis...Ch. 2 - Predict which of the following species is least...Ch. 2 - The molecules BH3 and SF6 and the ion SO42 exist...Ch. 2 - These are NOTlegitimate Lewisstructures (and...Ch. 2 - Fill in missing formal charges where needed (all...Ch. 2 - Below each structure in the previous question is a...Ch. 2 - Prob. 12ECh. 2 - Carbon monoxide (CO) is an example of an overall...Ch. 2 - Explain why this Lewis structure for CO is not as...Ch. 2 - Prob. 15ECh. 2 - Prob. 16ECh. 2 - Prob. 17ECh. 2 - Prob. 18ECh. 2 - Prob. 19E
Knowledge Booster
Similar questions
- Consider the molecules — BrF5. A. Draw the best Lewis structure for this molecule. Label any atoms with nonzero formal charge. B. Label each bond angle. As part of your answer be sure to include if it is more or less than the ideal bond angle. C. What is the electron geometry around the bromine atom? D. Are the bonds in the molecule polar? E. Is the overall molecule polar? — CH2 F2 . A. Draw the best Lewis structure for this molecule. B. Label each bond angle. Answers for A-D here: C. Redraw the shape of the molecule. Draw all dipoles. D. Is the overall molecule polar? — Consider the molecule CH2 CF2 . A. Draw the best Lewis structure for this molecule. B. Label each bond angle. C. Redraw the shape of the molecule (according to the exacting specifications of your instructor). Draw all dipoles. D. Is the overall molecule polar?arrow_forward1. For the oxalate ion C2O42- a. Draw the Lewis Structure. (Hint: the two carbon atoms are bonded to each other, and each carbon atom is bonded to two oxygen atoms.) b. Determine the EDG and MG for each carbon atom. c. Determine the bond angles around each carbon atom.arrow_forward|| Predicting deviations from ideal bond angles Consider the carbonyl fluoride (CF₂O) molecule. What is the central atom? Enter its chemical symbol. How many lone pairs are around the central atom? What is the ideal angle between the carbon-fluorine bonds? Compared to the ideal angle, you would expect the actual angle between the carbon-fluorine bonds to be ... 口。 (choose one) (choose one) about the same bigger smallerarrow_forward
- a. Predict the molecular structure and bond angles for ClO4¯. Approximate bond angles are sufficient. Molecular Structure = Bond Angles b. Predict the molecular structure and bond angles for PO3³-. = Approximate bond angles are sufficient. Molecular Structure = Bond Angles =arrow_forwardChange ethane into 1,2-ethanediol (HOCH2CH2OH) by removing one hydrogen atom from each carbon atom and replacing with a hydroxide (-OH) group. Build a model of the 1,2-ethanediol molecule. A. Identify the electron-pair and molecular geometry around each carbon and oxygen atom. B. What are the bond angles for H-C-H? For H-C-C? For C-C-O? For C-O-H? C. Draw two different structures of the molecule in which the –OH groups on the two carbon atoms have different orientations with respect to each other.arrow_forwardFor the following molecules, NF3 a. Give the total number of valence electrons in the moleculeb. Draw the lewis structurec. Name the molecule using the proper naming system for covalent molecules.d. Identify the electron geometry and the molecular geometry (shape)e. Give the electronic configurations for carbon, chlorine and fluorine. Identify anysimilarities.f. Find another molecule that has the same molecular geometry (the shape) as NF3arrow_forward
- 3. Select CO2 from the drop-down menu on the right of the simulation. Examine the model and real structures for CO2. a. Does each atom have the expected number of bonds? Explain your answer. b. Why is there agreement between the bond angle predicted by VSEPR theory and the actual bond angle?arrow_forward3. Draw the Lewis-dot structure for C4H10 below. Remember the Lewis-dot structure is the same as the Expanded structure. A. What is the main intermolecular force acting on this molecule? B. Is this molecule polar or nonpolar? Provide a brief explanation (2-3 sentences) for your answer.arrow_forwardHelp me pleasearrow_forward
- 2. The Lewis structure for the acetate ion [C₂H3O2]¹ is given below: le a. Read section 1.9. What is the geometry (shape) around each of the carbon atoms in the acetate ion - tetrahedral, trigonal planar or linear? b. Each of the oxygen atoms in the acetate ion have an octet. Draw all lone pairs of electrons in the acetate ion given above. c. Read section 1.7. Use electron pushing to generate a resonance structure of the acetate Lewis structure given above.arrow_forwardDetermine if the structural formula below are acceptable Lewis structures for organic compounds. Point out the problems in cases where structure is invalid. CH3 CH;-N-CH-CH3 ČH3 A. This structure is correct because the valance of Nitrogen is complete. B. This is not a correct Lewis structure because Nitrogen can accommodate more atoms. C. This is not a correct Lewis structure because Nitrogen has a charge if it does not have three bonds. D. This is a correct Lewis structure.arrow_forwardWrite the electron dot structure for ethylene, C2H4.a. What is the total number of available valence electrons?_____b. In the space below, write the atomic symbols, then add the bonding electron pairs. Finally distribute any non-bonding electron pairs.c. If there are not enough valence electrons remaining for the carbon atoms, consider the possibility of having a multiple bond between the two carbon atoms. If there is a double covalent bond between the carbon atoms (sharing of 4 valence electrons), can each carbon satisfy the Octet Rule?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY