
(a)
The numbers of atoms in Silicon FCC unit cell.
Answer to Problem 2.8P
The numbers of atoms in Silicon FCC unit cell is
Explanation of Solution
Given:
Silicon is atom at
Lattice parameter of silicon is
Density of the silicon atoms is
Calculation:
Draw the crystal structure of silicon atoms.
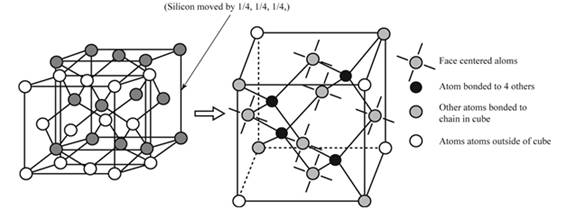
Figure (1)
FCC (Face-Centered structure) of Silicon has
Conclusion:
Therefore, the numbers of atoms in Silicon FCC unit cell is
(b)
The numbers of atoms per unit volume for the unit cell of Silicon.
Answer to Problem 2.8P
The numbers of atoms per unit volume for the unit cell of Silicon is
Explanation of Solution
Formula used:
Write the expression to find the number of atoms per unit volume.
Here,
Calculation:
Silicon has Face Centered Cubic (FCC) structure with
Substitute
Conclusion:
Therefore, the numbers of atoms per unit volume for the unit cell of Silicon is
(c)
The number of atoms per unit volume by using the density of silicon.
Answer to Problem 2.8P
The number of atoms per unit volume is
Explanation of Solution
Formula used:
Write the formula to find the atoms per unit volume.
Here,
Calculation:
The molecular weight of Silicon is
The density of Silicon is
Avogadro’s number is
Substitute
Conclusion:
Therefore, the number of atoms per unit volume is
Want to see more full solutions like this?
Chapter 2 Solutions
Materials Science And Engineering Properties
- Two hypothetical metals are created with different elements that have the same atomic mass (g/mole) and the same atomic radius. Metal A has a densityof 9.50 g/cm3 and metal B has a density of 8.73 g/cm3. If one of these metals has a BCC lattice structure and the other has an FCC lattice structure, identify the structure that corresponds to each of one of them. Justify your answer.arrow_forwardTwo hypothetical metals are created with different elements that have the same atomic mass (g/mole) and the same atomic radius. Metal A has a density of 9.50 g/cm3 and metal B has a density of 8.73 g/cm3. If one of these metals has a BCC lattice structure and the other has an FCC lattice structure, identify the structure that corresponds to each of one of them. Justify your answer.arrow_forwardCalculate the radius of the copper atom, given that copper has an FCC crystal structure, a density of 8.89 g/cm3 and an atomic mass of 63.55g/molarrow_forward
- * Your answer is incorrect. Iron has a BCC crystal structure, an atomic radius of 0.124 nm, and an atomic weight of 55.85 g/mol. Compute its theoretical density. 7.87 g/cm³arrow_forwardCalcium has an FCC crystal structure, density of 1.55 Mg/m3, and atomic massof 40.08 g/mole.a. Calculate the volume of the unit cell in cubic meters.b. Calculate the radius of the calcium atom.arrow_forwardExplain which kind of microstructures you expect to observe at room temperature if an iron-carbon alloy of eutectoid composition is cooled down following the red and blue curves. 800 727° 700 Coarse pearlite 600 a+ FeC Fine pearlite 500 y+ a+ Fe,C 400 Bainite 300 M5- 200 so - Mo 100 1 sec 1 min 1 hour 1 day 102 103 104 105 0.1 10 Time, secondsarrow_forward
 Materials Science And Engineering PropertiesCivil EngineeringISBN:9781111988609Author:Charles GilmorePublisher:Cengage Learning
Materials Science And Engineering PropertiesCivil EngineeringISBN:9781111988609Author:Charles GilmorePublisher:Cengage Learning
