
Concept explainers
Write structural formulas and line-angle formulas for the following
- (a) 2,2,4-Trimethylhexane
- (b) 2,2-Dimethylpropane
- (c) 3-Ethyl-2,4,5-trimethyloctane
- (d) 5-Butyl-2,2-dimethylnonane
- (e) 4-(1-Methylethyl)octane
- (f) 3,3-Dimethylpentane
- (g) trans-1,3-Dimethylcyclopentane
- (h) cis-1,2-Diethylcyclobutane
(a)
Interpretation:
The structural formula and line–angle formula of the given compound has to be written.
Concept Introduction:
Condensed structural formula:
Structural formula in which grouping of atoms are done and in which the central atoms along with the other atoms are connected to them are treated as group is known as Condensed structural formula.
Line–angle formula:
Structural formula where a line represent carbon‑carbon bond and the carbon atom is considered to be present in each point and the end of lines is known as Line-angle structural formula. This is a shorthand representation of an organic molecule with lines which represents its molecular bonding. In line–angle formula, hydrogen atoms are not shown.
Explanation of Solution
Given compound:
2,2,4-Trimethylhexane
Structural formula and line–angle formula:
From the name, it is known that the main carbon chain has six carbon atoms and it has two methyl groups at the second carbon and one methyl group at the fourth carbon.
The structural formula and line–angle formula of the given compound is,
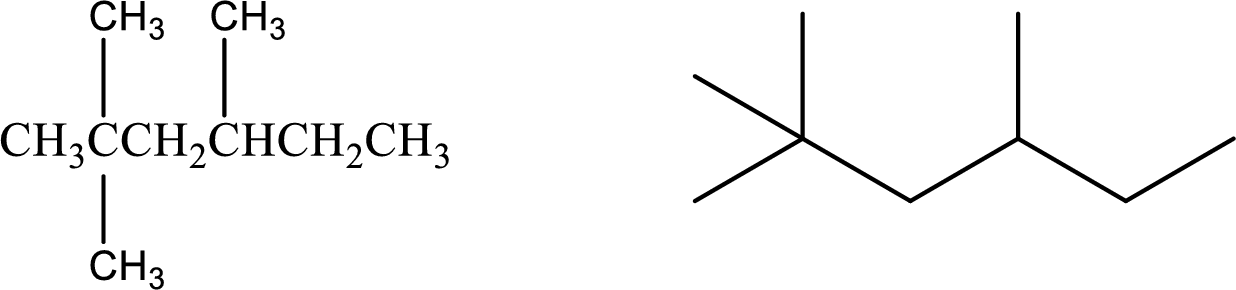
(b)
Interpretation:
The structural formula and line–angle formula of the given compound has to be written.
Concept Introduction:
Condensed structural formula:
Structural formula in which grouping of atoms are done and in which the central atoms along with the other atoms are connected to them are treated as group is known as Condensed structural formula.
Line–angle formula:
Structural formula where a line represent carbon‑carbon bond and the carbon atom is considered to be present in each point and the end of lines is known as Line-angle structural formula. This is a shorthand representation of an organic molecule with lines which represents its molecular bonding. In line–angle formula, hydrogen atoms are not shown.
Explanation of Solution
Given compound:
2,2-Dimethylpropane
Structural formula and line–angle formula:
From the given name, it is clear that the main carbon chain has three carbon atoms and two methyl groups are at the second carbon.
The structural formula and line–angle formula of the given compound is,

(c)
Interpretation:
The structural formula and line–angle formula of the given compound has to be written.
Concept Introduction:
Condensed structural formula:
Structural formula in which grouping of atoms are done and in which the central atoms along with the other atoms are connected to them are treated as group is known as Condensed structural formula.
Line–angle formula:
Structural formula where a line represent carbon‑carbon bond and the carbon atom is considered to be present in each point and the end of lines is known as Line-angle structural formula. This is a shorthand representation of an organic molecule with lines which represents its molecular bonding. In line–angle formula, hydrogen atoms are not shown.
Explanation of Solution
Given compound:
3-Ethyl-2,4,5-trimethyloctane
Structural formula and line–angle formula:
From the given name, it is clear that the main carbon chain has eight carbon atoms. Three methyl groups are at second, fourth and fifth carbon and one ethyl group is at third carbon.
The structural formula and line–angle formula of the given compound is,
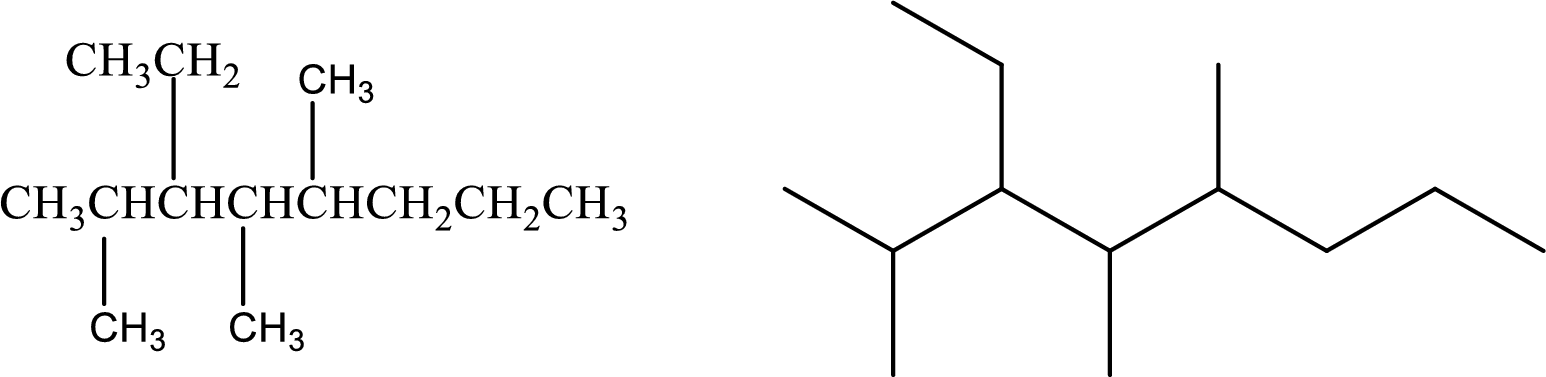
(d)
Interpretation:
The structural formula and line–angle formula of the given compound has to be written.
Concept Introduction:
Condensed structural formula:
Structural formula in which grouping of atoms are done and in which the central atoms along with the other atoms are connected to them are treated as group is known as Condensed structural formula.
Line–angle formula:
Structural formula where a line represent carbon‑carbon bond and the carbon atom is considered to be present in each point and the end of lines is known as Line-angle structural formula. This is a shorthand representation of an organic molecule with lines which represents its molecular bonding. In line–angle formula, hydrogen atoms are not shown.
Explanation of Solution
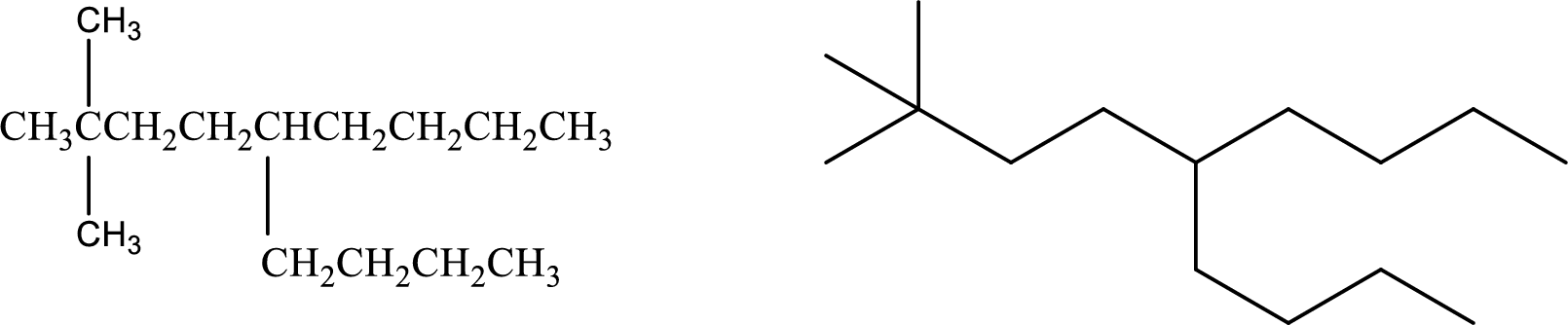
Given compound:
5-Butyl-2,2-dimethylnonane
Structural formula and line–angle formula:
From the given name, the main carbon chain nine carbon atoms. Two methyl groups are present at second carbon and one butyl groups is present at fifth carbon atom.
The structural formula and line–angle formula of the given compound is,
(e)
Interpretation:
The structural formula and line–angle formula of the given compound has to be written.
Concept Introduction:
Condensed structural formula:
Structural formula in which grouping of atoms are done and in which the central atoms along with the other atoms are connected to them are treated as group is known as Condensed structural formula.
Line–angle formula:
Structural formula where a line represent carbon‑carbon bond and the carbon atom is considered to be present in each point and the end of lines is known as Line-angle structural formula. This is a shorthand representation of an organic molecule with lines which represents its molecular bonding. In line–angle formula, hydrogen atoms are not shown.
Explanation of Solution
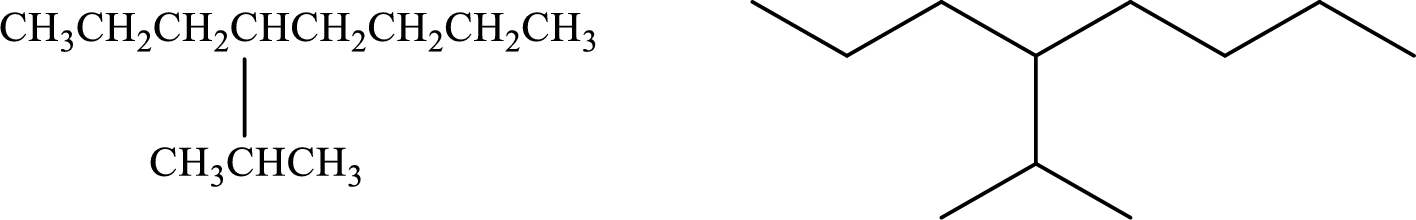
Given compound:
4-(1-Methylethyl)octane
Structural formula and line–angle formula:
From the given name, it is clear that the main carbon chain has eight carbon atoms. At the fourth carbon (1-methylethyl) group is attached.
The structural formula and line–angle formula of the given compound is,
(f)
Interpretation:
The structural formula and line–angle formula of the given compound has to be written.
Concept Introduction:
Condensed structural formula:
Structural formula in which grouping of atoms are done and in which the central atoms along with the other atoms are connected to them are treated as group is known as Condensed structural formula.
Line–angle formula:
Structural formula where a line represent carbon‑carbon bond and the carbon atom is considered to be present in each point and the end of lines is known as Line-angle structural formula. This is a shorthand representation of an organic molecule with lines which represents its molecular bonding. In line–angle formula, hydrogen atoms are not shown.
Explanation of Solution
Given compound:
3,3-Dimethylpentane
Structural formula and line–angle formula:
From the name, it is clear that the main carbon chain has five carbon atoms and two methyl groups are attached at the third carbon.
The structural formula and line–angle formula of the given compound is,

(g)
Interpretation:
The structural formula and line–angle formula of the given compound has to be written.
Concept Introduction:
Condensed structural formula:
Structural formula in which grouping of atoms are done and in which the central atoms along with the other atoms are connected to them are treated as group is known as Condensed structural formula.
Line–angle formula:
Structural formula where a line represent carbon‑carbon bond and the carbon atom is considered to be present in each point and the end of lines is known as Line-angle structural formula. This is a shorthand representation of an organic molecule with lines which represents its molecular bonding. In line–angle formula, hydrogen atoms are not shown.
Explanation of Solution
Given compound:
trans-1,3-Dimethylcyclopentane
Structural formula and line–angle formula:
From the name of the compound, it is clear that the main core of the compound is a five membered cyclic ring. Two methyl groups are attached at first and third carbon atom in such a way that both the methyl groups are in opposite direction of orientation with each other.
The structural formula and line–angle formula of the given compound is,
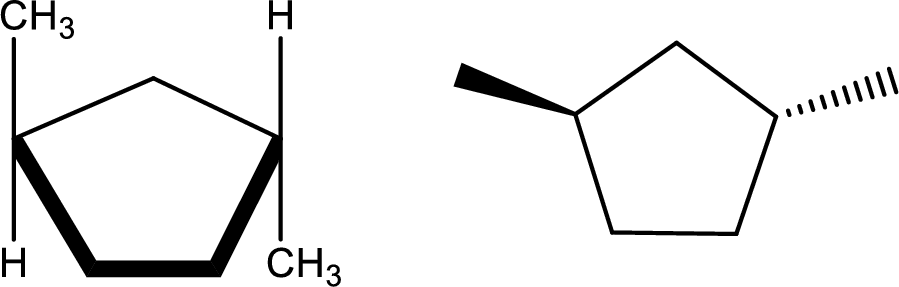
(h)
Interpretation:
The structural formula and line–angle formula of the given compound has to be written.
Concept Introduction:
Condensed structural formula:
Structural formula in which grouping of atoms are done and in which the central atoms along with the other atoms are connected to them are treated as group is known as Condensed structural formula.
Line–angle formula:
Structural formula where a line represent carbon‑carbon bond and the carbon atom is considered to be present in each point and the end of lines is known as Line-angle structural formula. This is a shorthand representation of an organic molecule with lines which represents its molecular bonding. In line–angle formula, hydrogen atoms are not shown.
Explanation of Solution
Given compound:
cis-1,2-Diethylcyclobutane
Structural formula and line–angle formula:
From the name of the compound, it is clear that the main core of the compound is a four membered cyclic ring. Two ethyl groups are attached at first and second carbon atom in such a way that both the ethyl groups are in same direction of orientation with each other.
The structural formula and line–angle formula of the given compound is,
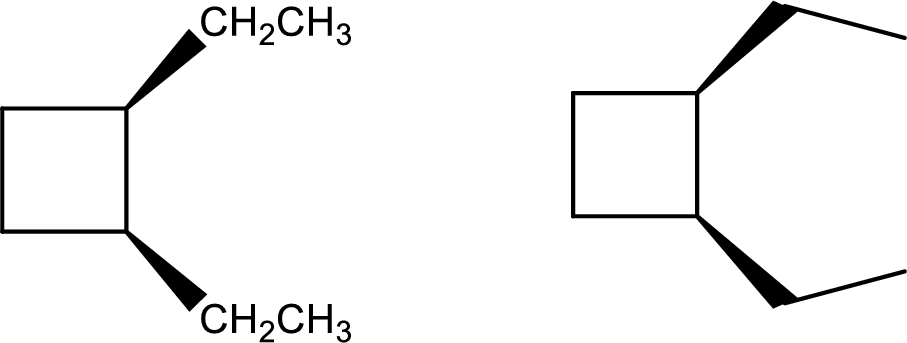
Want to see more full solutions like this?
Chapter 2 Solutions
Organic Chemistry
