
Pearson eText for Biochemistry: Concepts and Connections -- Instant Access (Pearson+)
2nd Edition
ISBN: 9780137533114
Author: Dean Appling, Spencer Anthony-Cahill
Publisher: PEARSON+
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 2, Problem 16P
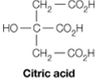
It is possible to make a buffer that functions well near pH 7 using citric acid, which contains only carboxylate groups. Explain
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
It is possible to make a buffer that functions well near pH7 using citric acid, which contains only carboxylate groups. Explain.
What kind of buffer would you make with a optimal activity at ph 4.30?
An appropriate biochemical buffer should have charges in both its
conjugate acid and conjugate base form. This avoids the presence
of a neutral form of the buffer that might be able slowly leak into
cells. Does unmodified dimethylpiperazine meet this criterion
when buffering at pH 8?
O Yes
No
What about buffering at pH 4? D Yes
No
Chapter 2 Solutions
Pearson eText for Biochemistry: Concepts and Connections -- Instant Access (Pearson+)
Ch. 2 - Suppose a chloride ion and a sodium ion are...Ch. 2 - Draw two different possible hydrogen-bonding...Ch. 2 - Prob. 3PCh. 2 - 4. What is the pH of each of the following...Ch. 2 - Prob. 5PCh. 2 - The weak acid HA is 2% ionized (dissociated) in a...Ch. 2 - 7. Calculate the pH values and draw the titration...Ch. 2 - What is the pH of the following buffer mixtures?...Ch. 2 - a. Suppose you wanted to make a buffer of exactly...Ch. 2 - Prob. 10P
Ch. 2 - You need to make a buffer whose pH is 7.0, and you...Ch. 2 - Describe the preparation of 2.00 L of 100 glycine...Ch. 2 - Carbon dioxide is dissolved in blood (pH 7.4) to...Ch. 2 - What is the molecular basis for the observation...Ch. 2 - The anno acid arginine ionizes according to the...Ch. 2 - It is possible to make a buffer that functions...Ch. 2 - A student is carrying out a biological preparation...Ch. 2 - Histidine is an amino acid with three titratable...Ch. 2 - Prob. 19PCh. 2 - A biochemical reaction takes place in a 1.00 ml...Ch. 2 - Is RNA-binding enzyme RNase A more likely to have...Ch. 2 - Consider a protein in which a negatively charged...Ch. 2 - Prob. 23PCh. 2 - Prob. 24P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- The pl of alkaline phosphatase is 4.5; the pl of the DEAE cellulose is 10.5. We used a buffer of pH 7.4 to run a DEAE-cellulose as anion exchange column? Explain how did this work?arrow_forwardWe usually say that a perfect buffer has its pH equal to its pKa. Give an example of a situation in which it would be advantageous to have a buffer with a pH 0.5 unit higher than its pKa.arrow_forwardIn ion-exchange chromatography, amino acids are separated on the basis of electro-static interactions and hydrophobic interactions with the resin. The chemical structure of the resin pol-ymer with a negatively charged sulfonic acid group is shown to the right. An elution profile for the aminoacids using this resin is given below. Based on the elution profile above, explain the following:arrow_forward
- Which of the following is TRUE if pH is higher than the pka of the buffer? O [weak acid] approximately 0 O [conjugate base] = [weak acid] [conjugate base] > [weak acid] O [conjugate base] < [weak acid]arrow_forwardBased on the intermediates outlined below, suggest why in different solvents and at different pH they are either soluble or insoluble.arrow_forwardAn amino acid mixture consisting of lysine, leucine, and glutamic acid is to be separated by ion-exchange chromatography, using a cationexchange resin at pH 3.5, with the eluting buffer at the same pH. Which of these amino acids will be eluted from the column first? Will any other treatment be needed to elute one of these amino acids from the column?arrow_forward
- When analyzing urine from a patient with kidney disease, which organicmolecules might be detected? Explain. Which biochemical test will be the most appropriate for detection of those molecules?arrow_forwardUsing DEAE-cellulose as ion exchange resin, indicate the starting and ending pH for the narrowest experimental pH range used to separate an amino acid mixture consisting of Gln, Leu and Lys Starting pH: _____ Ending pH: _____arrow_forwardDefine the following:- pH- Buffer- pKaarrow_forward
- give two disadvantages to using the biuret reaction to measure protein concentration compared to measuring the protein absorbance directly at 280 nm.arrow_forwardMixtures of amino acids can be analyzed by first separating the mixture into its components through ion‑exchange chromatography. Amino acids placed on a cation‑exchange resin containing sulfonate (−SO−3)(−SO3−) groups flow down the column at different rates because of two factors that influence their movement: (1) ionic attraction between the sulfonate residues on the column and positively charged functional groups on the amino acids, and (2) aggregation of nonpolar amino acid side chains with the hydrophobic backbone of the polystyrene resin. Note that the ionic attraction is more important than hydrophobicity for this column media. For each pair of amino acids, identify which will be eluted first from a cation‑exchange column using a pH 7.0pH 7.0 buffer.arrow_forwardWhat is the [CH3COO−]/[CH3COOH] ratio inan acetate buffer at pH 4.00?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

GCSE Chemistry - Acids and Bases #34; Author: Cognito;https://www.youtube.com/watch?v=vt8fB3MFzLk;License: Standard youtube license