
Interpretation: The amount of magnesium oxide formed by burning 8.0 g of magnesium should be determined.
Concept Introduction: In a chemical change, the
Answer to Problem 13STP
The amount of magnesium oxide formed by burning 8.0 g of magnesium is 13.0 g.
Explanation of Solution
| Mass of Magnesium (g) | Mass of Oxygen (g) | Mass of magnesium oxide (g) |
| 5 | 3.30 | 8.3 |
| 6.5 | 4.30 | 10.8 |
| 13.6 | 9.00 | 22.60 |
| 19.00 | 12.50 | 31.5 |
According to the law of conservation of mass, mass is conserved in any chemical or physical change. The mass of reactants is always equal to the mass of products during any
On plotting the mass of magnesium on the x-axis and mass of magnesium oxide on the y-axis, the plot obtained is:
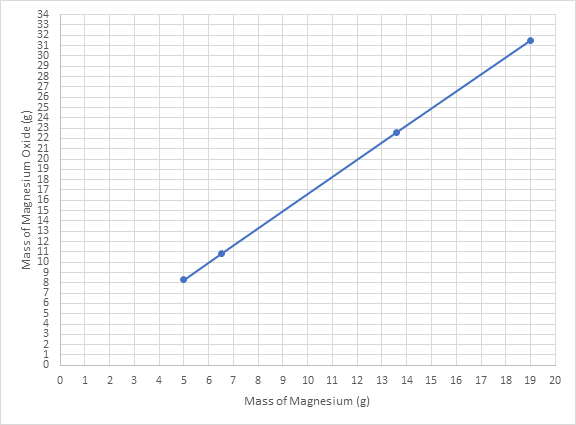
In order to determine the amount of magnesium oxide formed by burning 8.0 g of magnesium, the lines are drawn like this:
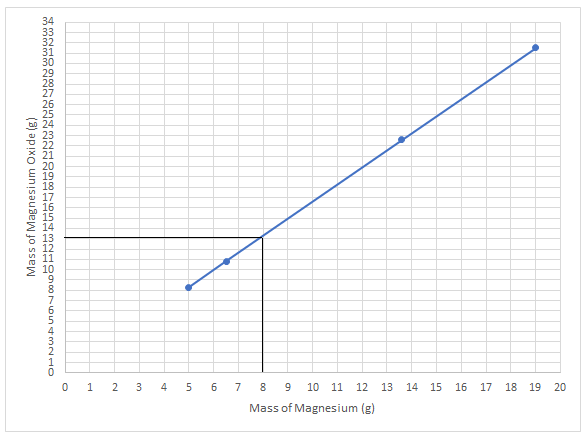
Thus, the amount of magnesium oxide formed by burning 8.0 g of magnesium is 13.0 g.
Chapter 2 Solutions
Chemistry 2012 Student Edition (hard Cover) Grade 11
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





